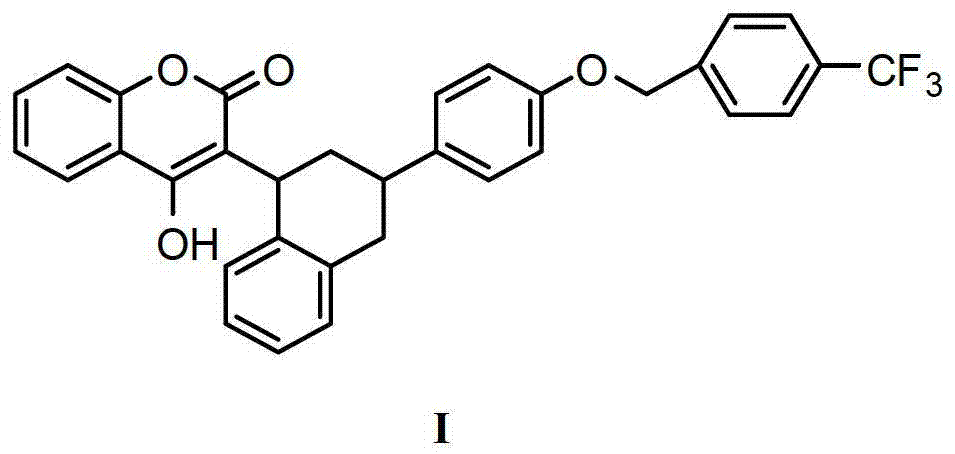
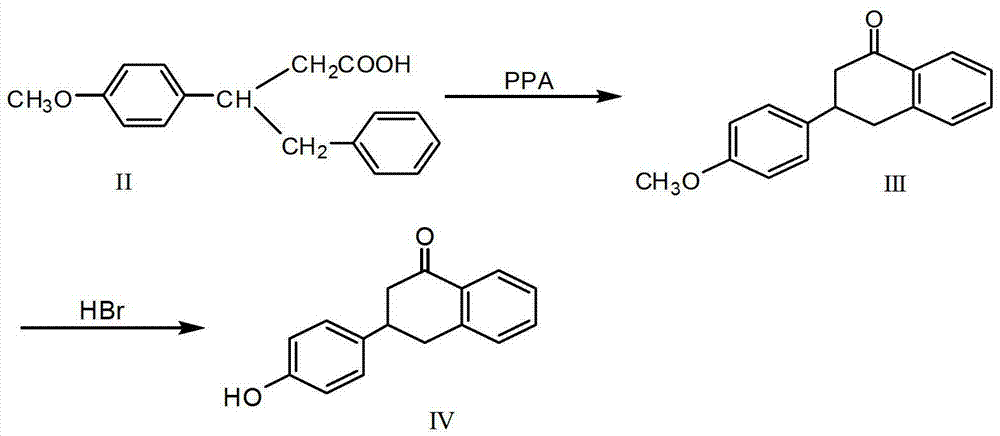
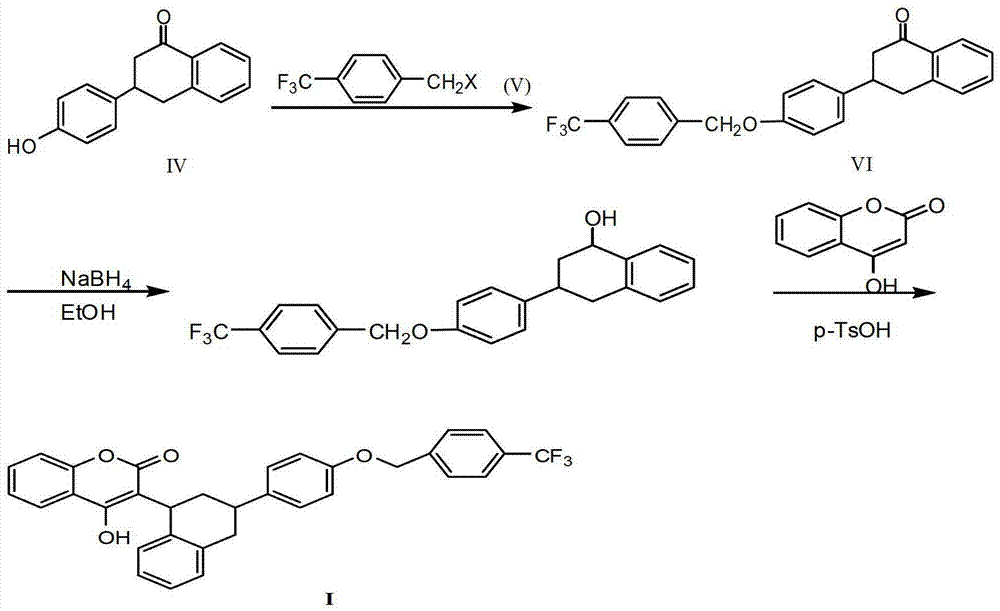
Synthesis methods of flocumafen and flocumafen intermediate
A synthesis method and intermediate technology, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve problems such as poor repeatability of the method, low product purity, and long reaction time, etc., to achieve the method Simple and effective, good atom economy, easy to react and controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035](1) Synthesis of 3-p-methoxyphenyl-1,2,3,4-tetralin-1-one (compound of formula III)
[0036] Take 10.8g of 3-benzyl-3-p-methoxyphenylpropionic acid (compound of formula II), dissolve it in 250mL of toluene to obtain a mixed solution, add 15.8g of benzenesulfonic acid to the mixed solution, reflux for 12h, and the reaction is complete. Evaporate the solvent to obtain the residue, add 60 mL of ethyl acetate to dissolve the residue after cooling to room temperature, then wash with water and 10% sodium carbonate solution in order, separate the organic phase, evaporate the solvent in the organic phase, and finally Recrystallized from methanol to obtain 7.2 g of 3-p-methoxyphenyl-1,2,3,4-tetrahydronaphthalene-1-one (compound of formula III), with a yield of 71%. mp (melting point) 102-105°C.
[0037] 1 HNMR (500MHz, CDCl 3 ) δ: 2.80~3.00 (m, 2H), 3.18~3.51 (m, 3H), 3.83 (s, 3H), 6.93 (d, J=8.5Hz, 2H), 7.24~7.55 (m, 5H), 8.10 (d, J=8.0Hz, 1H).
[0038] (2) Synthesis of 3-p...
Embodiment 2
[0049] (1) Synthesis of 3-p-methoxyphenyl-1,2,3,4-tetralin-1-one (compound of formula III)
[0050] Take 10.8g of 3-benzyl-3-p-methoxyphenylpropionic acid (compound of formula II), dissolve it in 300mL of dichloroethane, add 4.6g of trifluoroacetic acid, reflux for 20h, after the reaction is completed, evaporate the solvent to obtain Residue, the residue was cooled to room temperature, dissolved in 100 mL of ethyl acetate, washed with water and 10% sodium carbonate solution in sequence. The organic phase was separated, the solvent in the organic phase was evaporated, and methanol was recrystallized to obtain 6.6 g of 3-p-methoxyphenyl-1,2,3,4-tetrahydronaphthalene-1-one (compound of formula III), The yield is 65%. mp101-103°C.
[0051] Analysis data is with embodiment 1.
[0052] (2) Synthesis of 3-p-hydroxyphenyl-1,2,3,4-tetralin-1-one (compound of formula IV)
[0053] 3-p-methoxyphenyl-1,2,3,4-tetralin-1-one (compound of formula III) was subjected to demethylation reacti...
Embodiment 3
[0062] (1) Synthesis of 3-p-methoxyphenyl-1,2,3,4-tetralin-1-one (compound of formula III)
[0063] Take 10.8g of 3-benzyl-3-p-methoxyphenylpropionic acid (compound of formula II), dissolve it in 250mL of xylene, add 19.6g of trichloroacetic acid, and reflux for 5h. After the reaction is complete, evaporate the solvent to obtain a residue , the residue was cooled to room temperature, dissolved in 100 mL of ethyl acetate, washed with water and 10% sodium carbonate solution in sequence. The organic phase was separated, the solvent in the organic phase was evaporated, and methanol was recrystallized to obtain 7.0 g of 3-p-methoxyphenyl-1,2,3,4-tetralin-1-one (compound of formula III), The yield was 69%. mp101-103°C.
[0064] Analysis data is with embodiment 1.
[0065] (2) Synthesis of 3-p-hydroxyphenyl-1,2,3,4-tetralin-1-one (compound of formula IV)
[0066] 3-p-methoxyphenyl-1,2,3,4-tetralin-1-one (compound of formula III) was subjected to demethylation reaction to obtain c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com