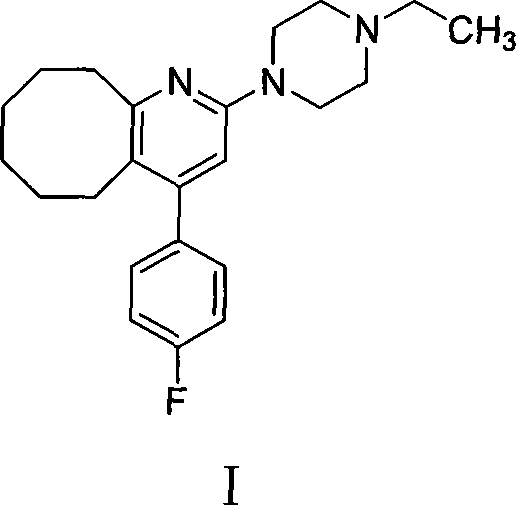
High-purity blonanserin and preparation method thereof
A technology of piperazinyl and compound, which is applied in the field of high-purity blonanserin and its preparation, and achieves the effects of low cost, favorable industrial production, and easy and controllable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
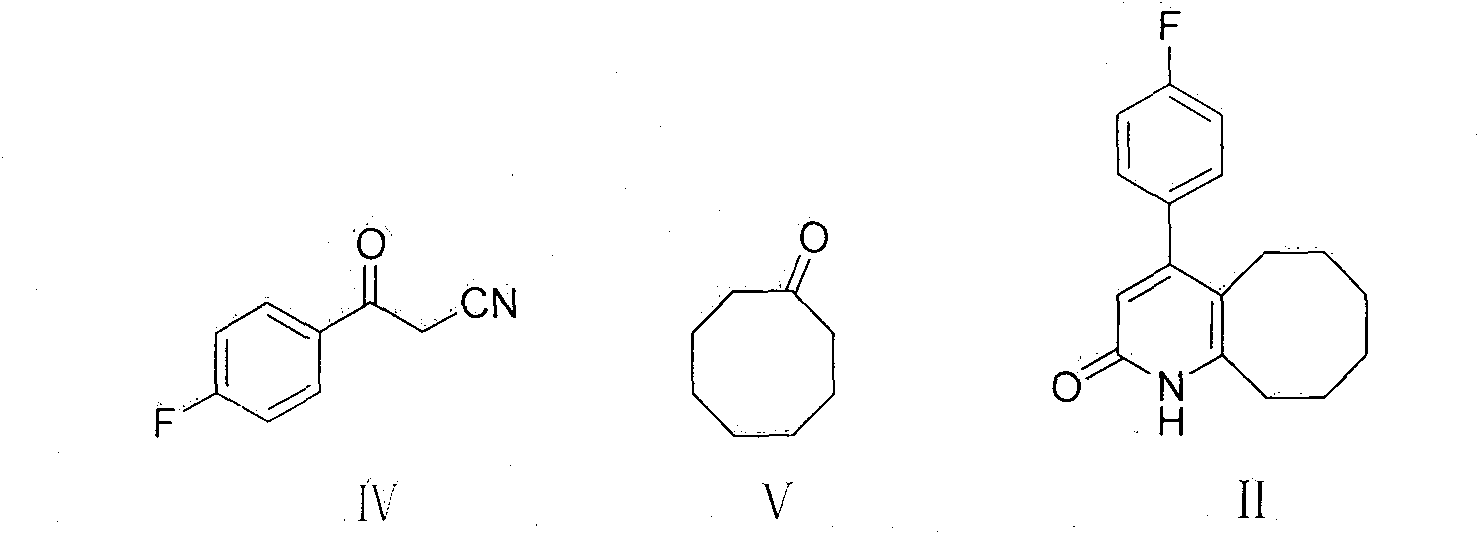
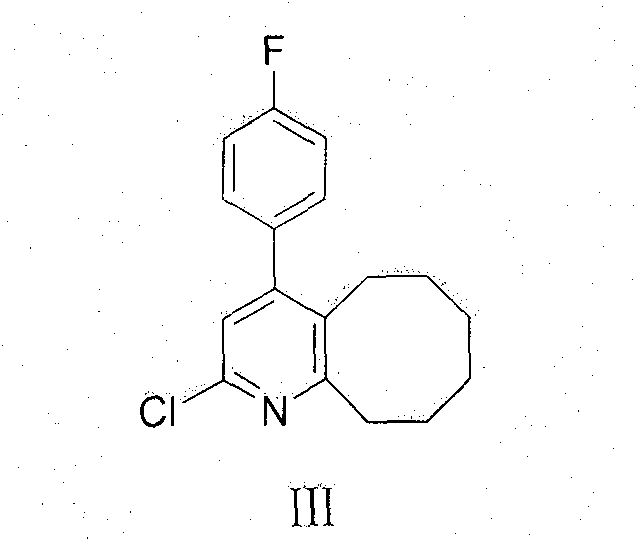
[0026] Add 163g of compound III, 380g of cyclooctanone, 5g of cyclohexylamine and RuH to a 2L three-neck flask 2 (PPh 3 ) 0.5g, heated to 160-170°C. The reaction was stirred for 5 hours, and the reaction was detected by TLC. After the reaction was completed, the temperature was lowered to 70° C., 100 ml of isopropanol was added, stirred while naturally cooling to room temperature, suction filtered, and the filter cake was dried to constant weight to obtain 211 g of the compound of formula II with a yield of 76%. Melting point: 236-239°C, HPLC purity 98%.
Embodiment 2
[0028] Remove 200 g of compound II obtained above, add 300 ml of phosphorus oxychloride, and heat to reflux for 2 hours. After cooling to room temperature, 2 L of chloroform was added to the mixture, and washed with saturated sodium carbonate. The organic phase was separated, dried with anhydrous sodium sulfate, the solvent was evaporated under reduced pressure, and the residue was purified with isopropanol to obtain 154 g of a solid product with a yield of 71%. Melting point: 111-112°C, HPLC purity 98%.
Embodiment 3
[0030] 140 g of Compound III obtained above, 150 g of methylpiperazine, 50 g of sodium iodide and 2 L of toluene were mixed and refluxed for 12 hours, cooled to room temperature, and washed with saturated sodium carbonate. The organic phase was separated, dried with anhydrous sodium sulfate, the solvent was evaporated under reduced pressure, and the residue was purified with isopropanol to obtain 123 g of a solid product with a yield of 78%. Melting point: 133-135°C, HPLC purity 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com