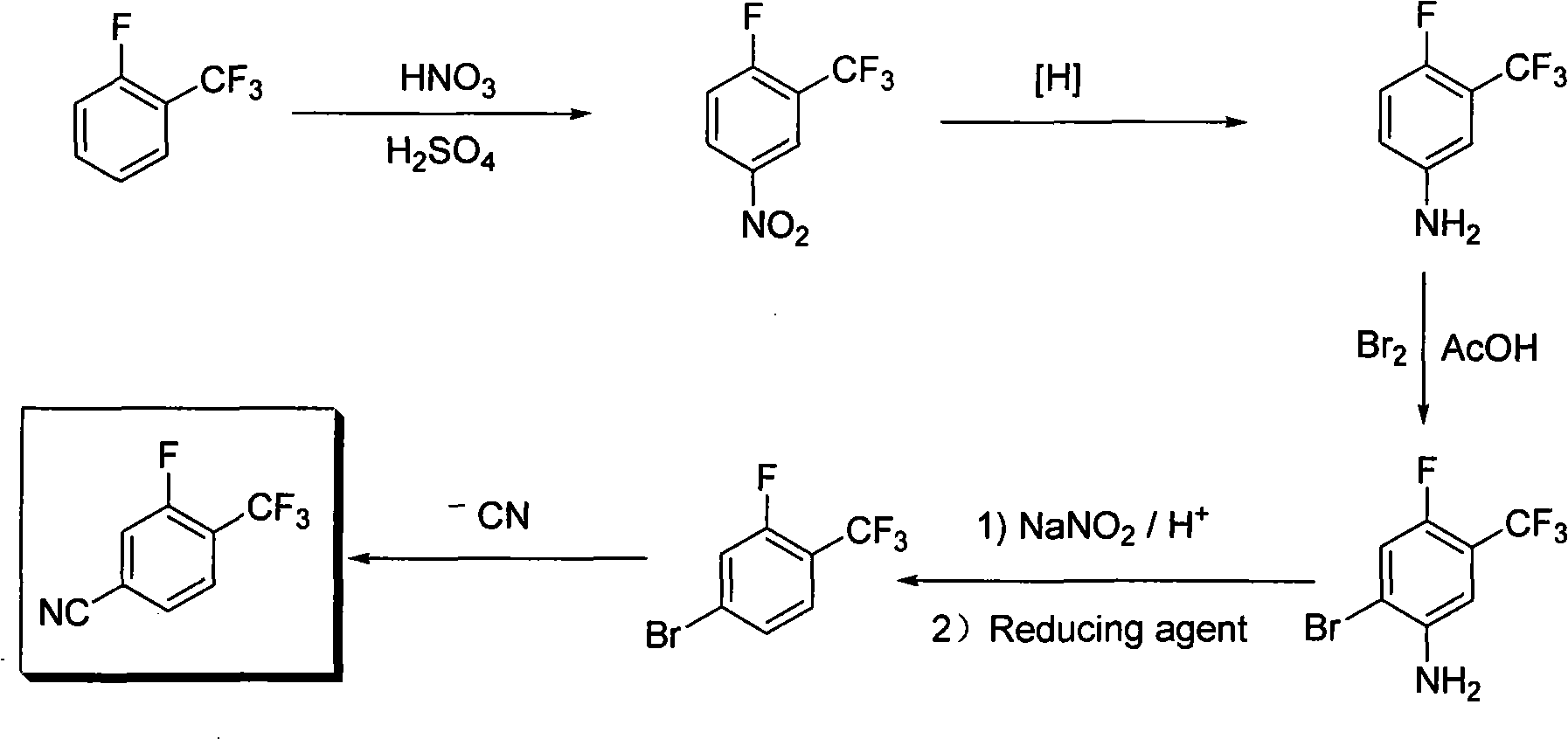
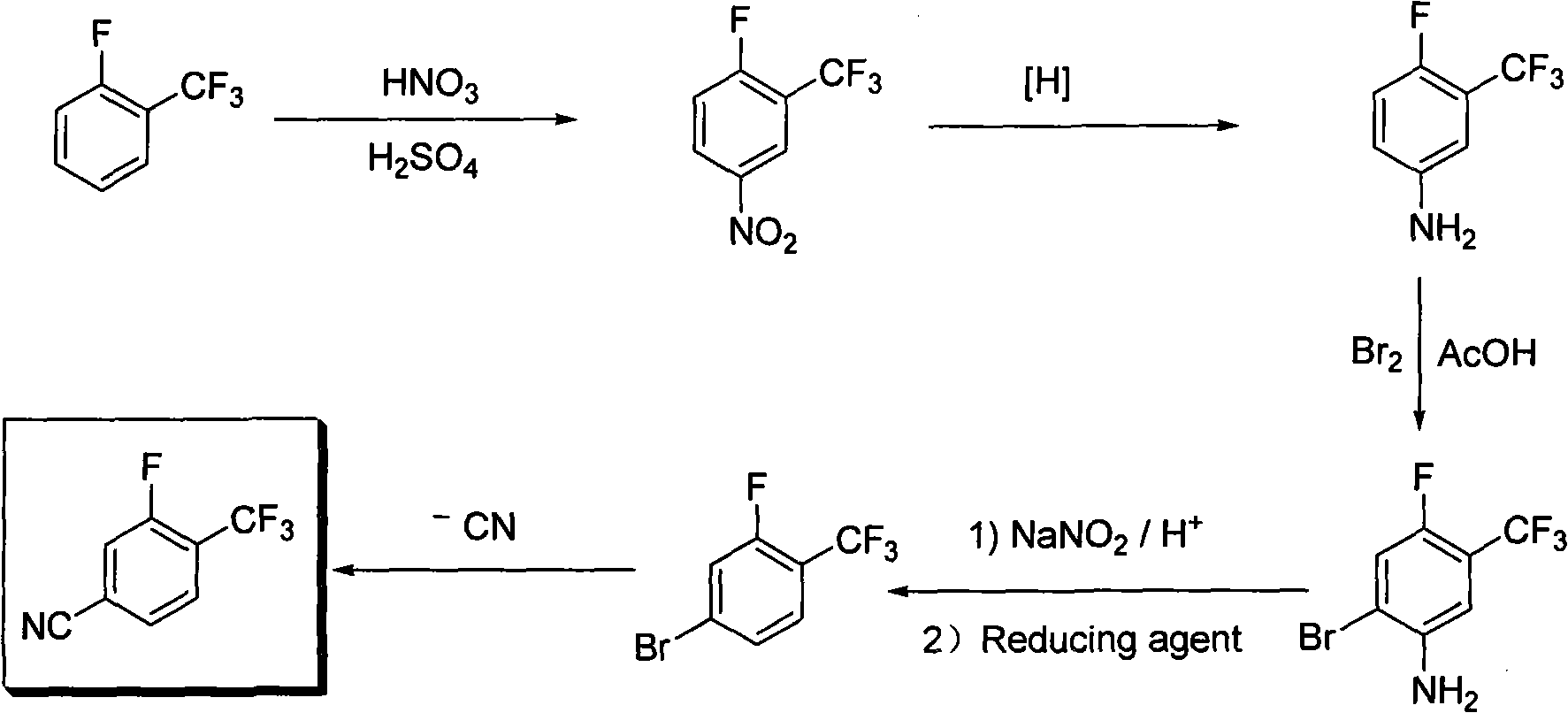
Method for preparing 3-fluor-4-trifluoromethylbenzonitrile
A technology of trifluoromethylbenzonitrile and o-fluorotrifluoromethylbenzene, which is applied in the field of preparation of 3-fluoro-4-trifluoromethylbenzonitrile, and can solve problems such as non-disclosure of technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: 3-trifluoromethyl-4-fluoronitrobenzene
[0015] A mechanical stirrer, a thermometer and a dropping funnel were installed on a two-liter three-neck reaction flask, and then 540mL of 98% sulfuric acid and 500.0g (3.049mol) of o-fluorobenzotrifluoride were added, and the temperature of the system did not change significantly. Use a beaker to prepare 144mL 98% nitric acid (3.354mol) and 180mL 98% sulfuric acid to make a uniform mixed acid, and then transfer it to the dropping funnel. Under stirring, slowly add the mixed acid dropwise into the reaction mixture, the temperature rises significantly during the dropwise addition, and the temperature of the reaction system is controlled between 10-20°C, and it takes about 3 hours to complete the dropwise. Stirring was continued for 0.5 hours after dropping. After the system was left to stand for stratification, the water layer was separated and extracted once with dichloromethane. The dichloromethane solution was mixe...
Embodiment 2
[0016] Example 2: 3-trifluoromethyl-4-fluoroaniline
[0017] Mechanical stirring, reflux condenser, thermometer and dropping funnel are installed on the four-necked reaction flask of two liters, then add 1087 milliliters of water, 333.7g iron powder (5.958mol) and 42.8g ammonium chloride (0.793mol), heat Warm to reflux. Heating was stopped after reflux, and 425 g (2.03 mol) of 3-trifluoromethyl-4-fluoronitrobenzene was slowly added dropwise to the system, and the system was kept under reflux slowly, and the dropwise addition was completed after 2.0 hours. Reflux was continued for 3.5 hours. The reflux device was changed to a steam distillation device, and the organic layer was separated from the water to obtain 421.3 g of colorless liquid 3-trifluoromethyl-4-fluoroaniline, with a yield of more than 100% and a purity of 99.12% (GC).
Embodiment 3
[0018] Example 3: 2-Bromo-4-fluoro-5-trifluoromethylaniline
[0019] Install a mechanical stirrer, a thermometer and a dropping funnel on a 5-liter three-neck reaction flask, then add 2450 mL of acetic acid and 490.0 g (2.737 mol) of 3-trifluoromethyl-4-fluoroaniline into the reaction flask. Under stirring, 481.0 g (3.0 mol) of bromine was added dropwise through the dropping funnel, and the temperature of the system was controlled at 10-20°C. After the dropwise addition was complete (about 2 hours), stirring was continued at room temperature for 2 hours. Pour the system into 5L of water, brown oil can be seen, adjust the pH of the system to about 7 with saturated sodium carbonate aqueous solution, a solid appears, filter with suction to obtain a brownish yellow solid 2-bromo-4-fluoro-5-trifluoromethane Aniline 600g, yield 85%, purity 93.35% (GC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com