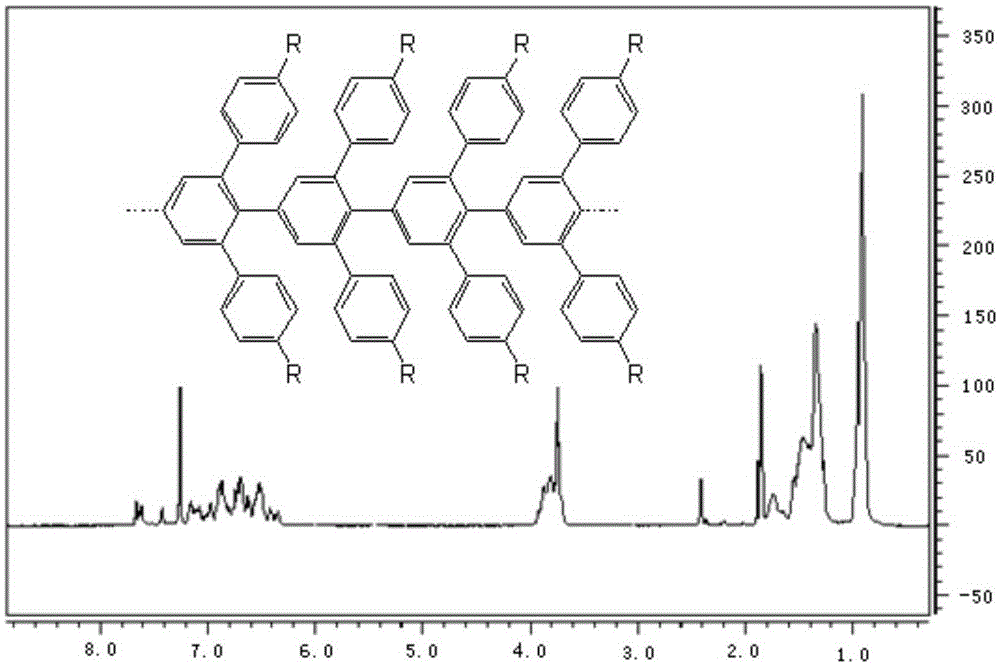
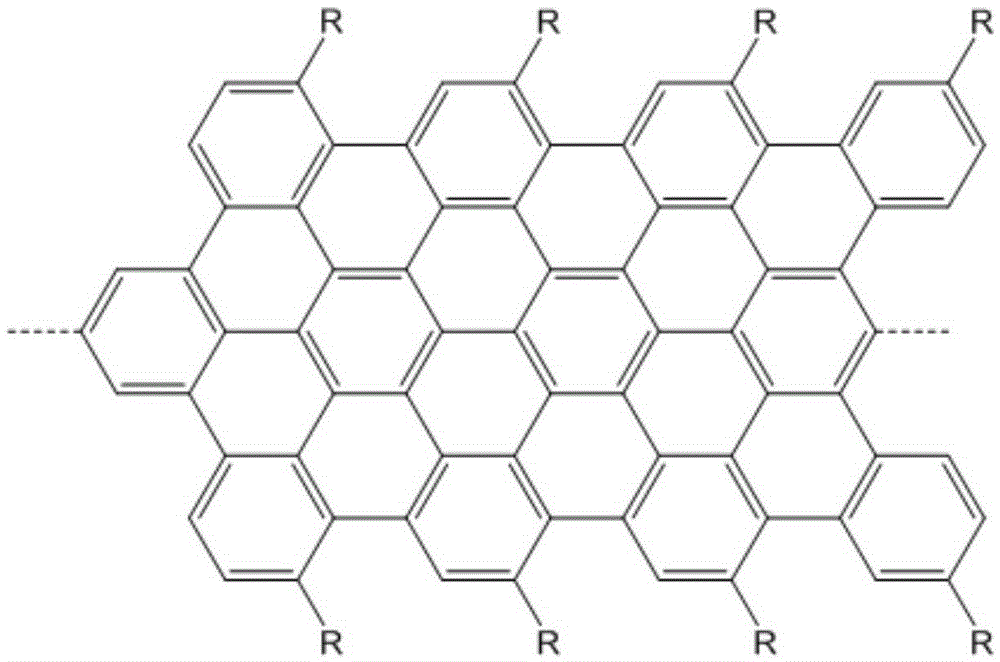
Graphene nanoribbon and synthesis method and application thereof
A graphene nanoribbon and synthesis method technology, applied in the field of functional materials, can solve the problems of low yield, high cost, complicated route, etc., and achieve the effects of high mobility, reliable process, and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A graphene nanoribbon, its synthetic method comprises the steps:
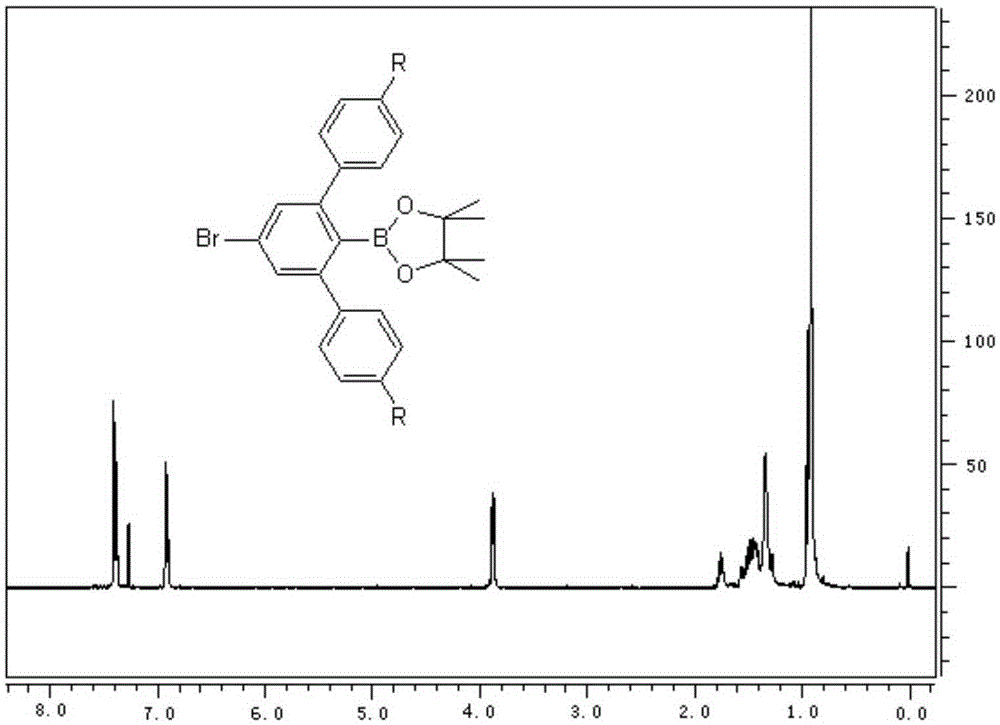
[0035] (1) Synthesis of 3,5-bis(4-(2-ethylhexyloxy)-benzene)-4-anol borate-1-bromobenzene:
[0036] The synthetic route is shown in the following formula:
[0037]
[0038] The specific operation is:
[0039] Add 60mL of 4-(2-ethylhexyloxy)-bromobenzene Grignard reagent (0.5M, 30mmol, tetrahydrofuran (THF) solution) into a 250mL three-necked flask, heat to 80°C and reflux, take 2,4,6 -Tribromoiodobenzene (10mmol, 4.407g) was dissolved in 30mLTHF, poured into the constant pressure funnel and added dropwise, dripped for 1h, refluxed at 80°C for 3h, cooled to room temperature, added 60mL of THF, cooled to -65°C, injected 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 10mL, remove the cooling device, and react overnight at natural temperature; after the reaction, add water, use Extract with dichloromethane, add magnesium sulfate to dry, filter with suction, spin dry, and drain; silica gel powder ...
Embodiment 2
[0054] A graphene nanoribbon, its synthetic method comprises the steps:
[0055] (1) Synthesis of 3,5-bis(4-octylbenzene)-4-anol borate-1-bromobenzene:
[0056] The synthetic route is shown in the following formula:
[0057]
[0058] The specific operation is:
[0059] Add 60mL of 4-octyl-bromobenzene Grignard reagent (0.5M, 30mmol, THF solution) into a 250mL three-necked flask, heat to reflux at 80°C, and take 2,4,6-tribromoiodobenzene (10mmol, 4.407g ) was dissolved in 30mL THF, poured dropwise into a constant pressure funnel, dripped for 1h, refluxed at 80°C for 3h, cooled to room temperature, added 60mL THF, cooled to -65°C, injected 2-isopropoxy-4,4 , 10mL of 5,5-tetramethyl-1,3,2-dioxaborolane, remove the cooling device, and react overnight at natural temperature; after the reaction, add water, extract with dichloromethane, add magnesium sulfate to dry, Suction filtration, spin-drying, and suction-drying; the silica gel powder was passed through the column, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com