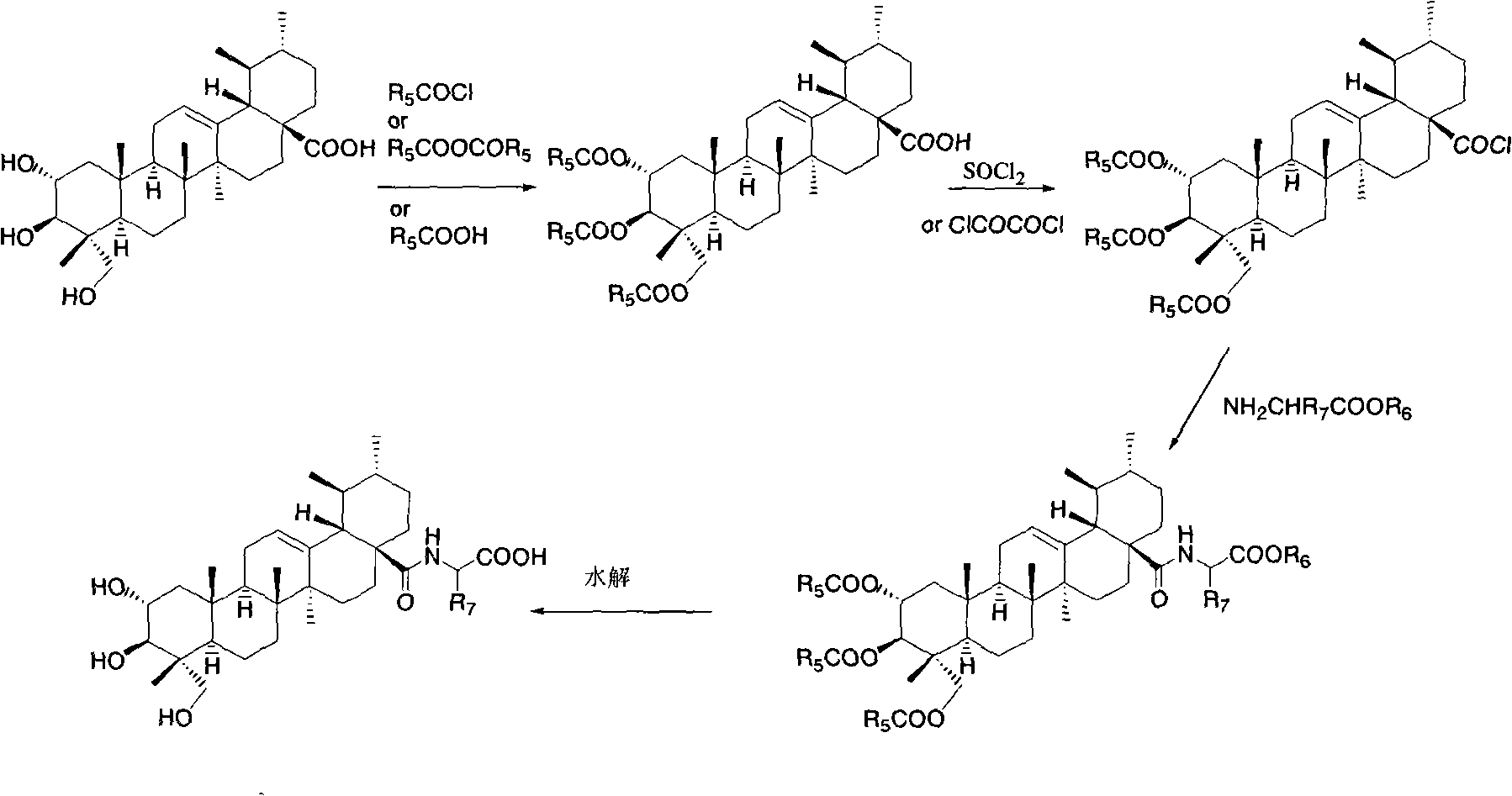
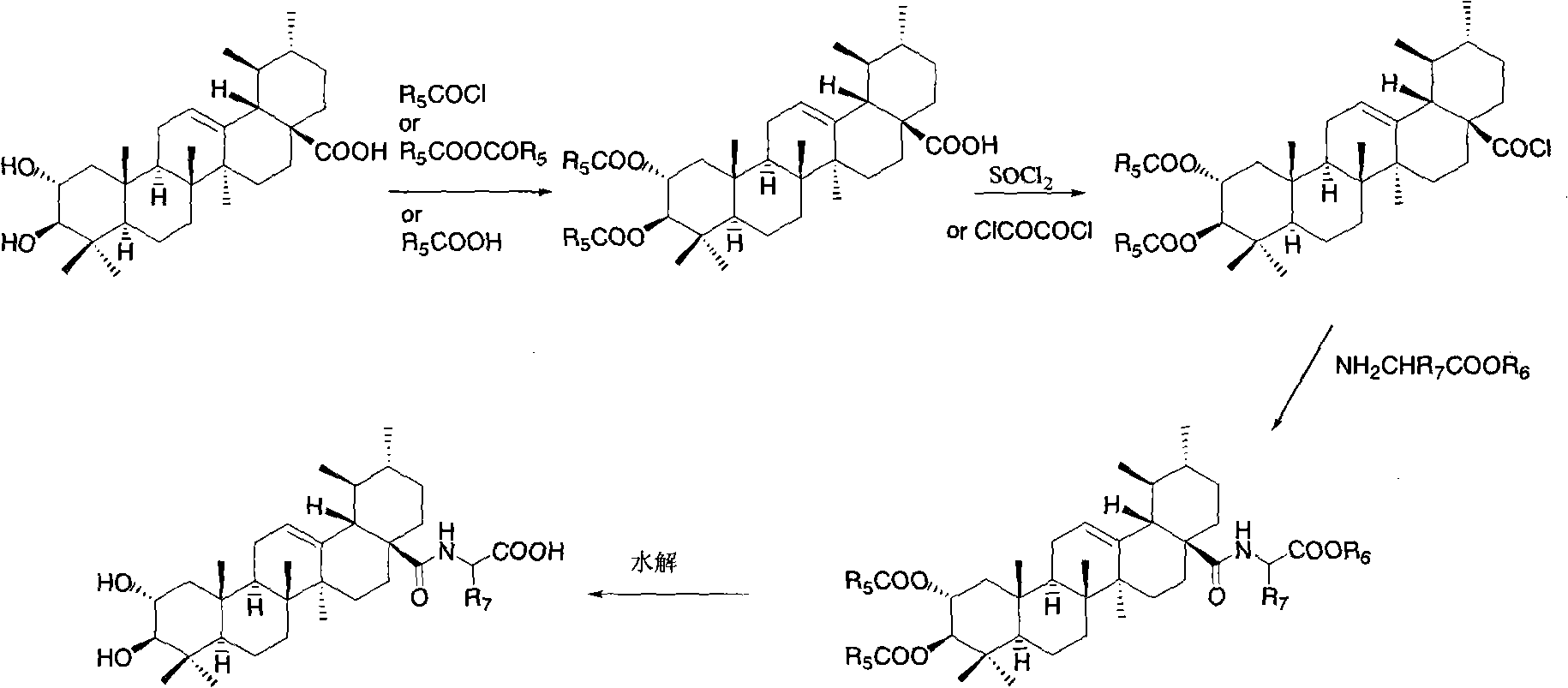
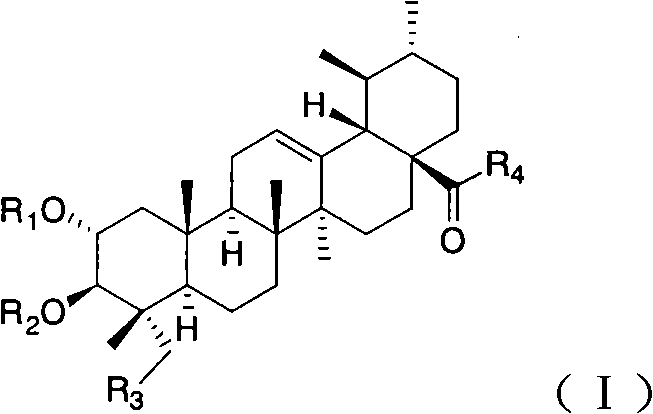
Bearberry type pentacyclic triterpenes amino acid derivates, method for preparing same and pharmaceutical use thereof
A technology of amino acid and amino acid ester, which is applied in the field of natural medicine and medicinal chemistry, and can solve the problems of low bioavailability of preparations, poor water solubility of corosolic acid and asiatic acid, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Synthesis of 2a, 3β, 23-triacetoxy asiatic acid
[0104] Add asiatic acid (0.20g, 0.40mmol) and acetic anhydride (76mg, 0.74mmol) into pyridine (10ml), and stir overnight at room temperature. After the reaction solution was diluted with ethyl acetate, it was washed successively with water, 1N dilute hydrochloric acid, saturated sodium bicarbonate solution and saturated brine, the organic phase was dried, filtered, concentrated, and subjected to flash column chromatography (petroleum ether / ethyl acetate: 10 / 1V / V) to give 2a, 3β, 23-O-triacetyl asiatic acid (170mg, 78%). M.p.93-95°C.
[0105] ESI-MSm / z: 637[M+Na] + .
[0106] 1 H-NMR (CDCl 3 , 300MHz): 0.78, 0.85, 0.88, 0.94, 1.07, 1.11(each 3H, s), 1.98, 2.02, 2.08(each 3H, s), 2.19(1H, d, J=11.3Hz), 3.58(1H, d,J=11.8Hz), 3.85(1H,d,J=11.8Hz), 5.07(1H,d,J=10.3Hz), 5.12-5.17(1H,m), 5.25(1H,t).
Embodiment 2
[0108] Synthesis of N-(2a,3β,23-triacetoxyarbutan-12-en-28-amide)-glycine methyl ester
[0109] Dissolve 2a, 3β, 23-triacetoxy asiatic acid (0.25g, 0.40mmol) in refined and dry dichloromethane (20ml), add oxalyl chloride (0.8ml) dropwise under ice-cooling, and stir at room temperature for 24 hours. Dichloromethane was distilled off under reduced pressure, then refined and dry dichloromethane (20×3) was added, dissolved, and distilled off to obtain 2a, 3β, 23-triacetoxy asiaticoyl chloride. Glycine methyl ester hydrochloride (0.15g, 1.20mmol) and DMAP (0.03g, 0.22mmol) were dissolved in dry dichloromethane, and the dichloromethane solution of the above-mentioned freshly prepared triacetylated asiaticoyl chloride was added dropwise, after dropping, stirred at room temperature for 12 hours, the reaction solution was washed successively with 1N aqueous hydrochloric acid solution and saturated brine, the organic layer was dried, filtered, concentrated, and subjected to flash column...
Embodiment 3
[0113] Synthesis of N-(2a,3β,23-triacetoxyarbutan-12-en-28-amide)-L-alanine methyl ester
[0114] Referring to the method of Example 2, N-(2a, 3β, 23-triacetoxyurbutane was prepared with 2a, 3β, 23-triacetoxy asiatic acid and L-alanine methyl ester hydrochloride -12-ene-28-amide)-L-alanine methyl ester. M.p. 220-222°C.
[0115] ESI-MS m / z: 700.5[M+H] + .
[0116] 1 H-NMR (CDCl 3 , 300MHz): 0.70(3H, s), 0.88(6H, s), 0.96(3H, s), 1.09(9H, s), 1.98, 2.02, 2.08(each 3H, s), 3.57(1H, d, J=11.8Hz), 3.75(3H, s), 3.84(1H, d, J=11.8Hz), 4.45-4.49(1H, m), 5.07(1H, d, J=10.3Hz), 5.12-5.17( 1H, m), 5.40(1H, t), 6.57(1H, d, J=5.9Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com