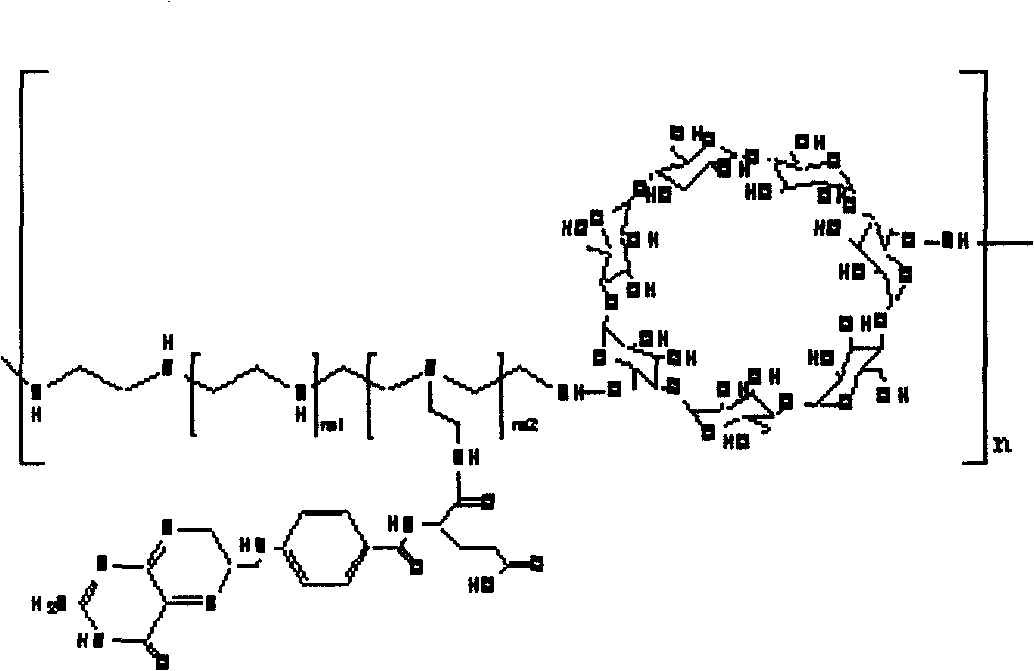
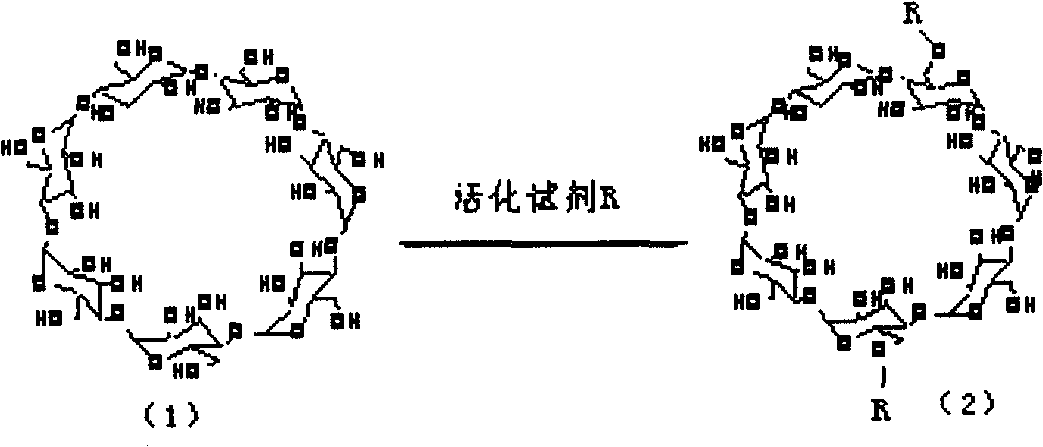
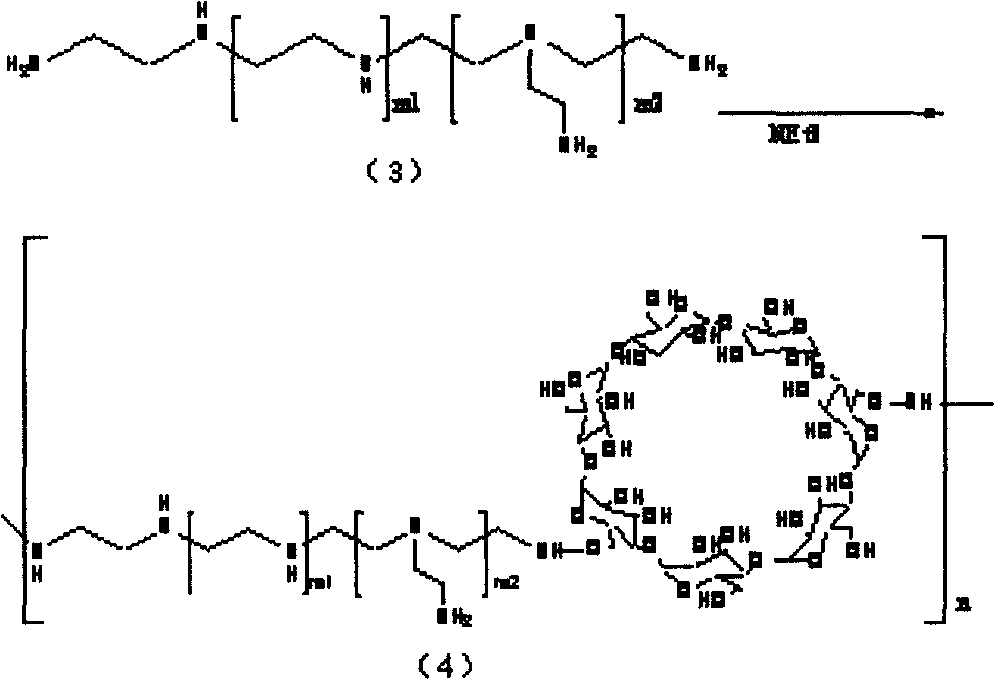
Polycation transgene vector and method for synthesizing same
A technology of transgenic carrier and polycation, which is applied in the field of polycation carrier and synthesis, can solve unrelated problems and achieve the effects of prolonging the action time, improving transfection efficiency, and tumor targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Activation of cyclodextrin: take 1.9 grams of α-cyclodextrin, add 10 ml of dimethyl sulfoxide to dissolve it, and activate 10 grams of hydroxyl linker N, N'-disuccinimidyl sulfate Dissolve it in 20 ml of dimethyl sulfoxide, add the linking agent into the cyclodextrin solution in the dark under the protection of nitrogen, add 200 microliters of catalyst triethylamine, and stir for 120-240 minutes to react. After the reaction was completed, it was precipitated in refrigerated ether to obtain a white precipitate, which was filtered and dissolved in dimethyl sulfoxide for storage.
[0035] 2) Coupling of polyethyleneimine: take 2.5 grams of activated cyclodextrin, dissolve 9 grams of polyethyleneimine with a molecular weight of 600 in 20 ml of dimethyl sulfoxide, and protect the mixture from light under nitrogen protection. Add dropwise to the above-mentioned cyclodextrin solution, the dropping speed is slow, and the addition is completed in 90-120 minutes, and 300 micro...
Embodiment 2
[0038] 1) Activation of cyclodextrin: 2.1 g of β-cyclodextrin, dissolved in 15 ml of dimethyl sulfoxide, 15 g of linker N, N'-carbonyldiimidazole, which activates the hydroxyl group, was dissolved in 2 ml of dimethyl In the sulfoxide, add the linking agent into the cyclodextrin solution in the dark under the protection of nitrogen, add 300 microliters of catalyst triethylamine, and stir for 120-240 minutes to react. After the reaction was completed, it was precipitated in refrigerated ether to obtain a white precipitate, which was filtered and dissolved in dimethyl sulfoxide for storage.
[0039] 2) Coupling of polyethyleneimine: take 2.5 grams of activated cyclodextrin, dissolve 12 grams of polyethyleneimine with a molecular weight of 1200 in 20 ml of dimethyl sulfoxide, and remove it from light under nitrogen protection. Add dropwise to the cyclodextrin solution at a slow rate, and finish adding in 90-120 minutes, add 300 microliters of catalyst triethylamine, and continue t...
Embodiment 3
[0042] 1) Activation of cyclodextrin: 2.5 grams of γ-cyclodextrin, add 20 milliliters of dimethyl sulfoxide to dissolve it, and dissolve 20 grams of benzotriazole carbonate, a linker for activating hydroxyl groups, in 20 milliliters of dimethyl In the base sulfoxide, under the protection of nitrogen, add the linking agent into the cyclodextrin solution in the dark, add 150 microliters of catalyst triethylamine, and stir for 120-240 minutes to react. After the reaction was completed, it was precipitated in refrigerated ether to obtain a white precipitate, which was filtered and dissolved in dimethyl sulfoxide for storage.
[0043] 2) Coupling of polyethyleneimine: take 2.5 grams of activated cyclodextrin, dissolve 15 grams of polyethyleneimine with a molecular weight of 2000 in 30 milliliters of dimethyl sulfoxide, and put it dropwise under nitrogen protection in the dark. Add to the above cyclodextrin solution, the dropping speed is slow, and the addition is completed in 90-12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com