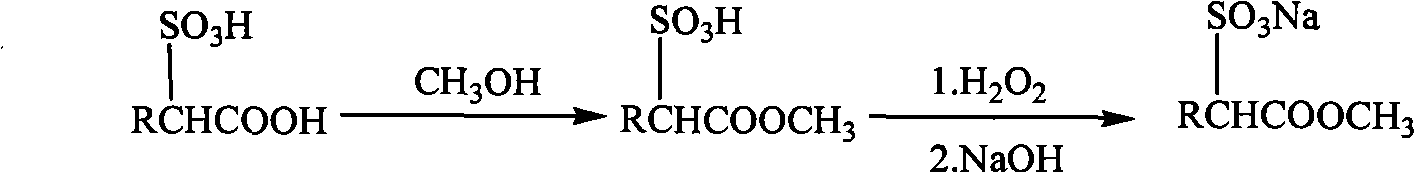
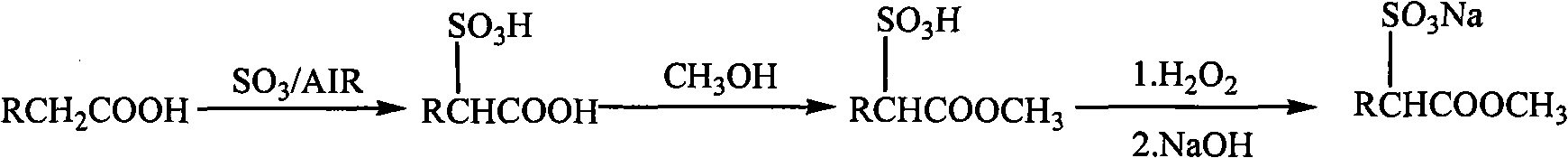Method for preparing fatty acid methyl ester sulphonic salt with low-disodium salt content by using earlier sulfonation and later esterification technique
A technology of fatty acid methyl ester sulfonate and its manufacturing method, which is applied in the fields of sulfonate preparation and organic chemistry, and can solve the problems of α-sulfo fatty acid without breakthrough progress, lack of countermeasures and systematic research, etc., and achieve product quality Good, the effect of reducing separation steps and shortening the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 12
[0017] Example 1 Synthesis of sodium dodecanoic acid methyl sulfonate
[0018] In a 250mL reaction device with heating, stirring and temperature control system, 28g (0.1mol) of α-sulfododecanoic acid prepared by sulfonation was added, and the molar ratio of α-sulfo fatty acid: methanol = 1:30 Add 96g (3mol) of methanol to the system, reflux for 10h; then use H in the methanol solution phase 2 O 2 Bleach until the Klett color is not greater than 50; continue to massage the molar ratio of sodium hydroxide:α-methyl sulfododecanoate=1:1 in the methanol solution phase and add dropwise a 20% mass fraction of sodium hydroxide methanol solution to the system, Neutralize at room temperature, adjust the pH of the system to 7; remove the methanol by rotary evaporation to obtain 31.8 g of sodium dodecanoate sulfonate product, in which the content of the active ester sodium salt is 93.6%, and the content of the disodium salt is less than 0.5%.
Embodiment 2 12
[0019] Example 2 Synthesis of sodium dodecanoic acid methyl sulfonate
[0020] In a 500L reaction device with heating, stirring and temperature control system, add 56kg (0.2kmol) of α-sulfododecanoic acid prepared by sulfonation in advance, molar ratio α-sulfododecanoic acid: methanol=1 :40 Add 256kg (8kmol) of methanol to the system, reflux for 7h; then use H in the methanol solution phase 2 O 2 Bleach until the Klett color is not greater than 50; continue to massage the molar ratio of sodium hydroxide:α-methyl sulfododecanoate = 1:1 into the system by adding 10% mass fraction of methanol solution of sodium hydroxide, Neutralize at 30°C, adjust the system pH to 7.5; evaporate and remove methanol to obtain 63.6 kg of sodium dodecanoate sulfonate product, in which the content of the active ester sodium salt is 92.8%, and the content of the disodium salt is less than 0.5%.
Embodiment 3 16
[0021] Example 3 Synthesis of sodium hexadecanoic acid methyl sulfonate
[0022] In a 250mL reaction device with heating, stirring and temperature control system, 33.6g (0.1mol) of α-sulfohexadecanoic acid prepared by sulfonation was added, and the molar ratio of α-sulfohexadecanoic acid: methanol = 1:20 Add 64g (2mol) of methanol to the system, reflux for 6h; then use H in the methanol solution phase 2 O 2 Bleach until the Klett color is not more than 50; continue to massage the molar ratio of sodium hydroxide:α-methyl sulfohexadecanoate = 1:1 into the system by adding 15% mass fraction of sodium hydroxide methanol solution, Neutralize at 35°C and adjust the pH of the system to 8; the methanol is removed by rotary evaporation to obtain 37.4 g of sodium hexadecanoate sulfonate product, in which the content of the active ester sodium salt is 86.4%, and the content of disodium salt is 1.56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com