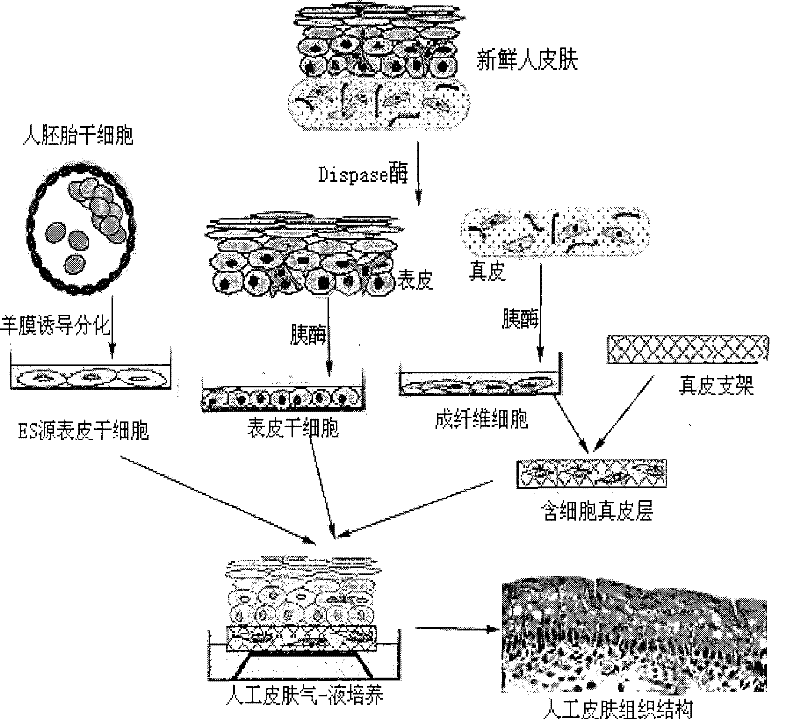
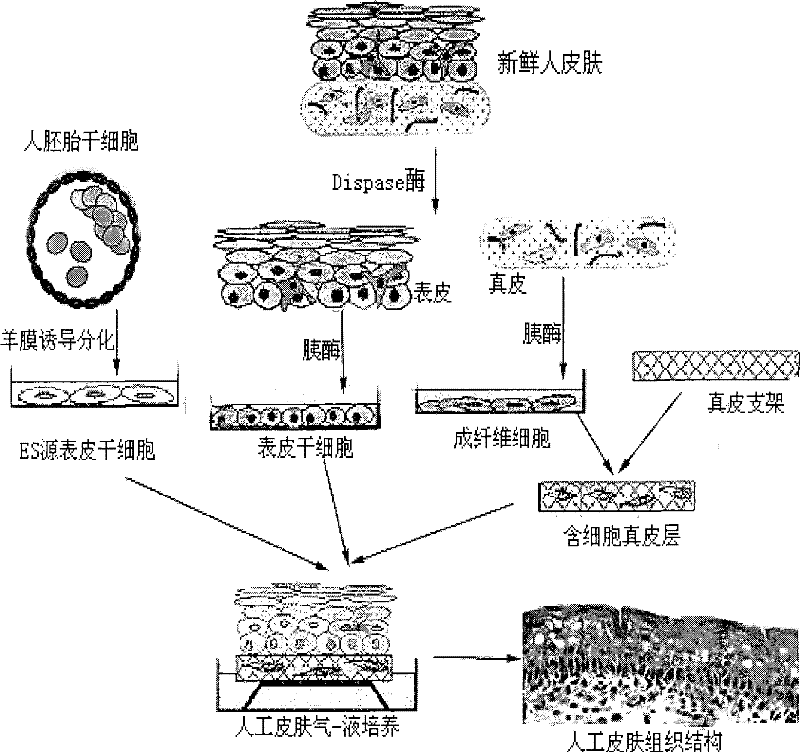
Method for preparing full-thickness skin for toxicity test by stem cell raft type cultivation
A full-thickness skin and stem cell technology, applied in the field of toxicology tests, can solve the problems of short skin function maintenance time, inapplicability to chronic toxicity tests, long culture period, etc., to achieve small batch-to-batch differences, complete morphology and tissue structure, and maintain long time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Include the following steps in turn:
[0044] 1. Preparation and modification of polymer dermal scaffolds
[0045] 1) Preparation of PHBV scaffold:
[0046] (a) Dissolving an appropriate amount of PHBV powder in chloroform to make a solution with a concentration of 120-130g / L, then adding 940g of sodium chloride (NaCl) to each liter of the solution as a porogen, fully mixing to obtain a thick slurry;
[0047] (b) Pour the thick slurry into a circular metal mold with a hole diameter of 0.8 to 1.0 cm. After about 80% of the chloroform solvent is volatilized, take out the forming bracket from the mold, and then place it in a fume hood at room temperature for 48 hours to dry. Vacuum drying to remove residual solvent;
[0048] (c) Immerse the formed scaffold in deionized water, change the water once every 6 hours, and change the water 8 times within 48 hours until the porogen is filtered out, dry at room temperature and vacuum dry for 24 hours to make a PHBV porous three-...
Embodiment 2
[0071] 1. Preparation and modification of polymer dermal scaffolds
[0072] 1) Preparation of PHBV scaffold:
[0073] (a) dissolving an appropriate amount of PHBV powder in chloroform to make a solution with a concentration of 130g / L, then adding 930g of sodium chloride (NaCl) to each liter of solution as a porogen, fully mixing to obtain a thick slurry;
[0074] (b) Pour the thick slurry into 0.15-0.2ml of a circular metal mold with a diameter of 1.0cm. After the solvent volatilizes, take out the forming bracket from the mold, place it in a fume hood at room temperature for 54 hours, and dry it in vacuum to remove the residual solvent. ;
[0075] (c) Immerse the formed scaffold in deionized water, change the water once every 7 hours, change the water 7 times within 49 hours until the porogen is filtered out, dry at room temperature and then vacuum dry for 24 hours to make a PHBV porous three-dimensional scaffold ;
[0076] (d) Soak the PHBV porous three-dimensional scaffol...
Embodiment 3
[0094] 1. Preparation and modification of polymer dermal scaffolds
[0095] 1) Preparation of PHBV scaffold:
[0096] (a) Dissolving an appropriate amount of PHBV powder in chloroform to make a solution with a concentration of 130g / L, then adding 940g of sodium chloride (NaCl) to each liter of solution as a porogen, fully mixing to obtain a thick slurry;
[0097] (b) Pour the thick slurry into 0.15-0.2ml of a circular metal mold with a diameter of 1.0cm. After the solvent volatilizes, take out the forming bracket from the mold, place it in a fume hood at room temperature for 54 hours, and dry it in vacuum to remove the residual solvent. ;
[0098] (c) Immerse the formed scaffold in deionized water, change the water once every 8 hours, and change the water 7 times within 56 hours until the porogen is filtered out, dry at room temperature and vacuum dry for 24 hours to make a PHBV porous three-dimensional scaffold ;
[0099] (d) Soak the PHBV porous three-dimensional scaffold...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com