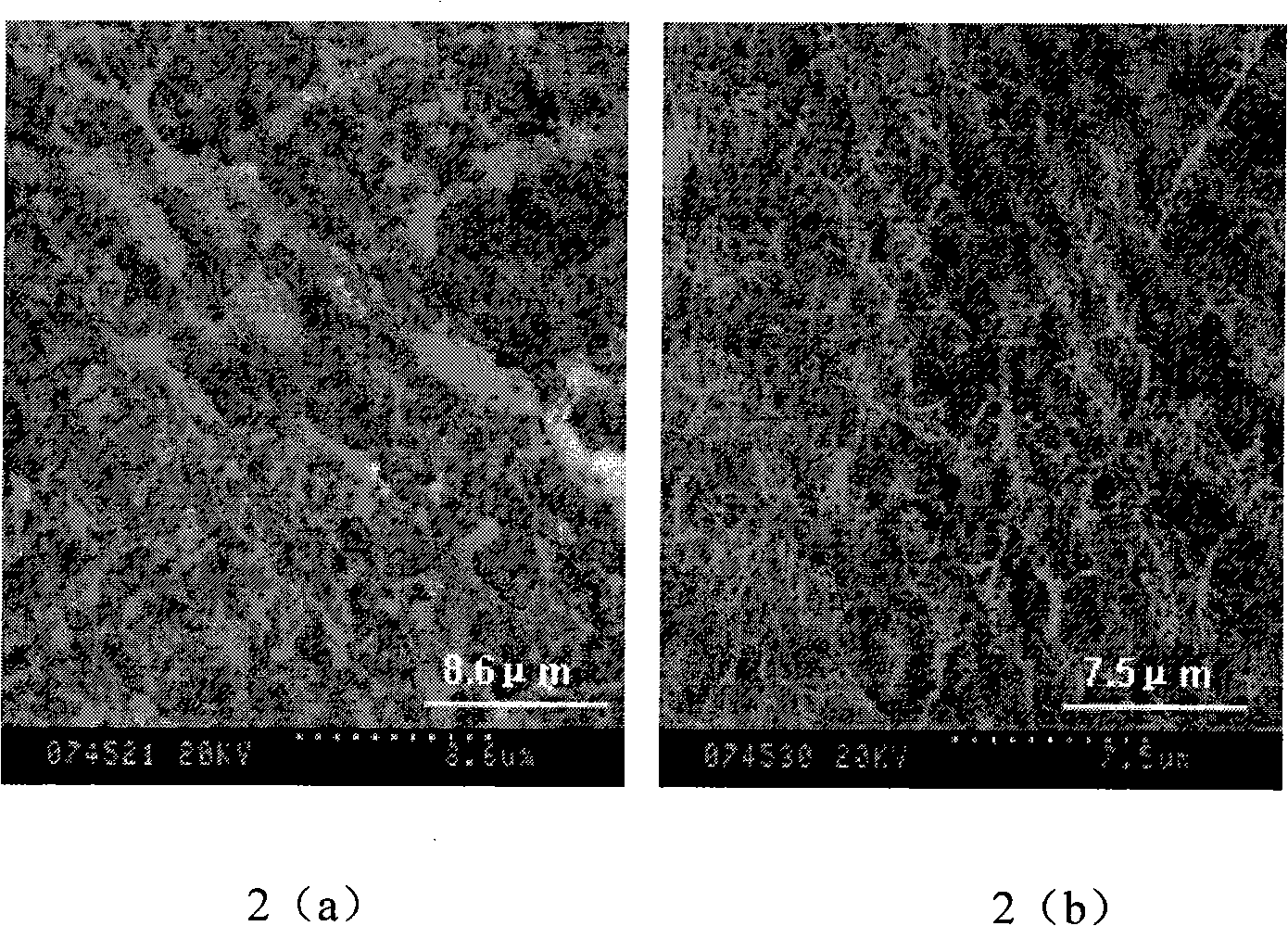
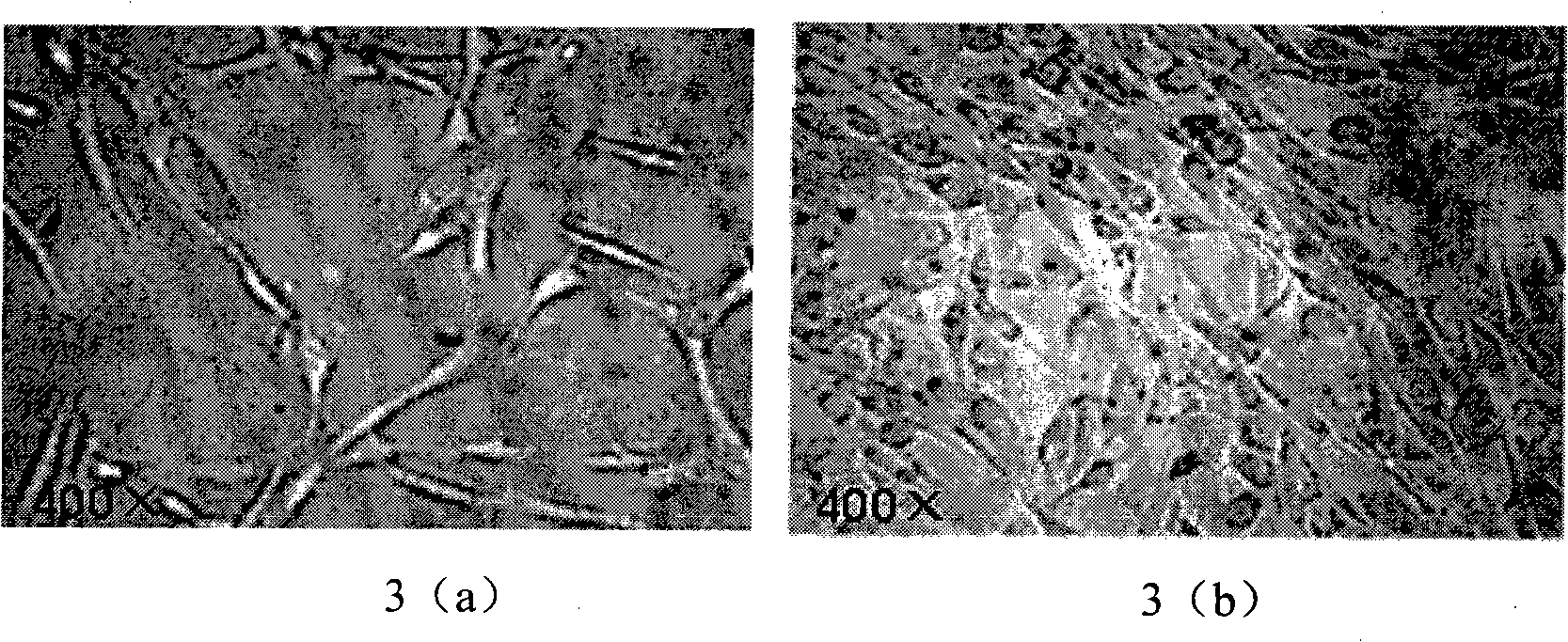
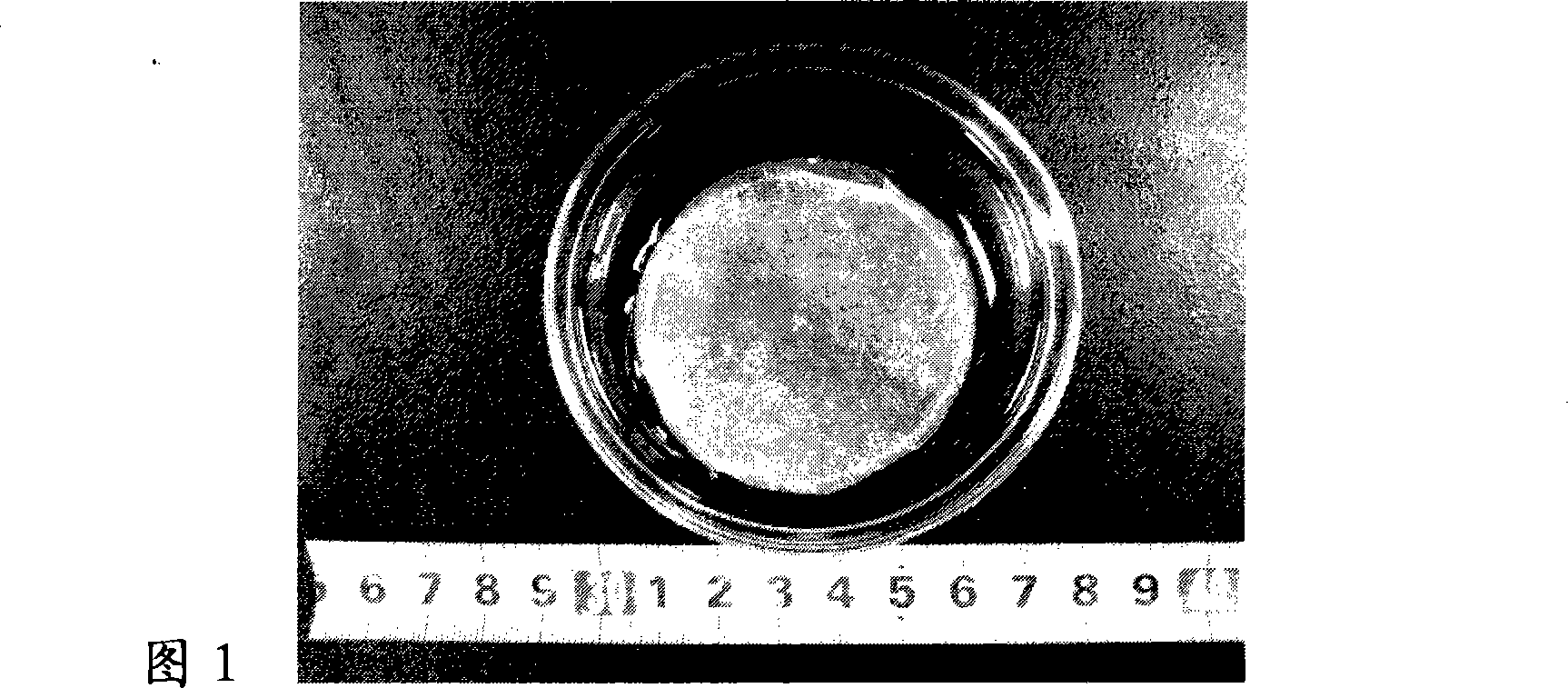
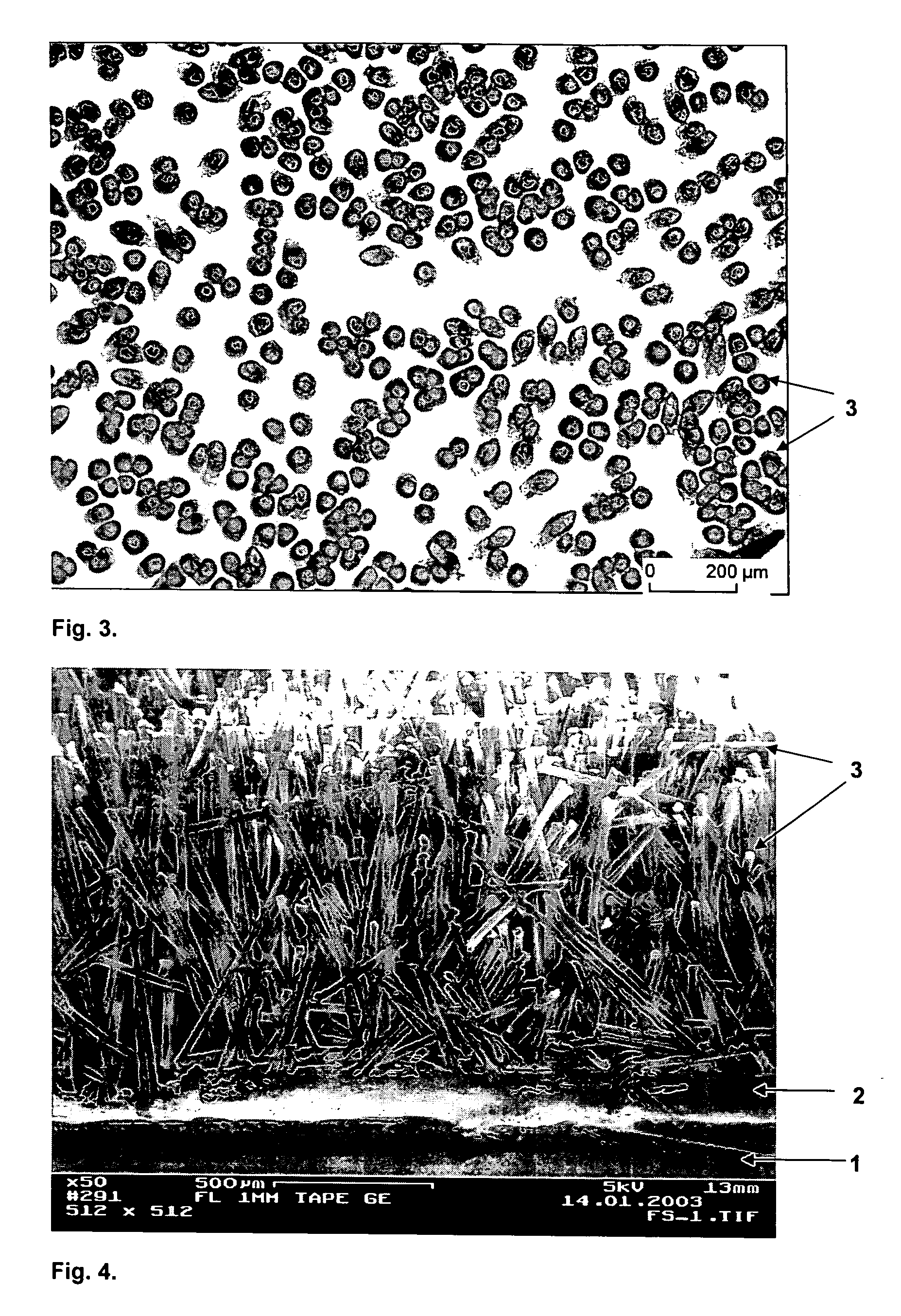
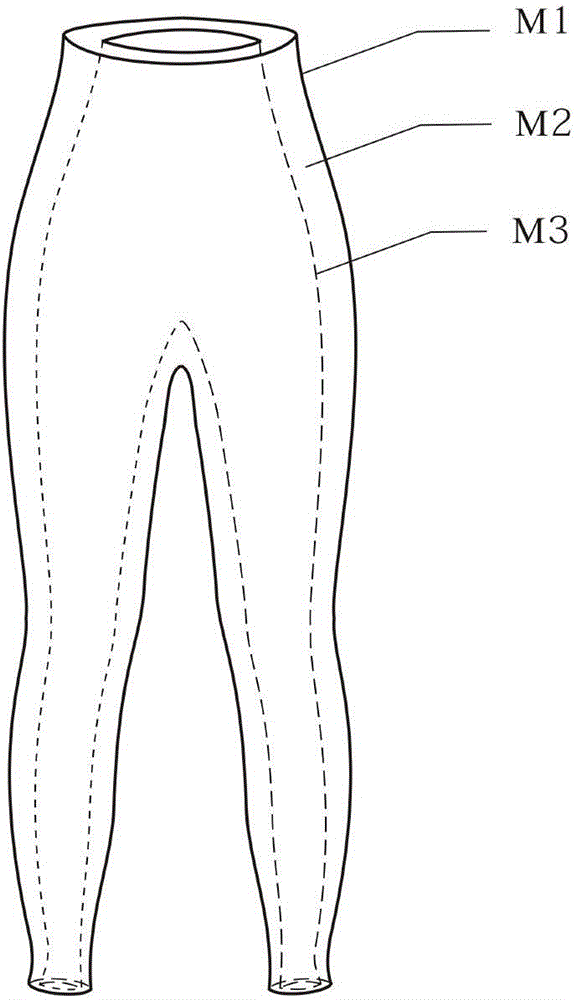
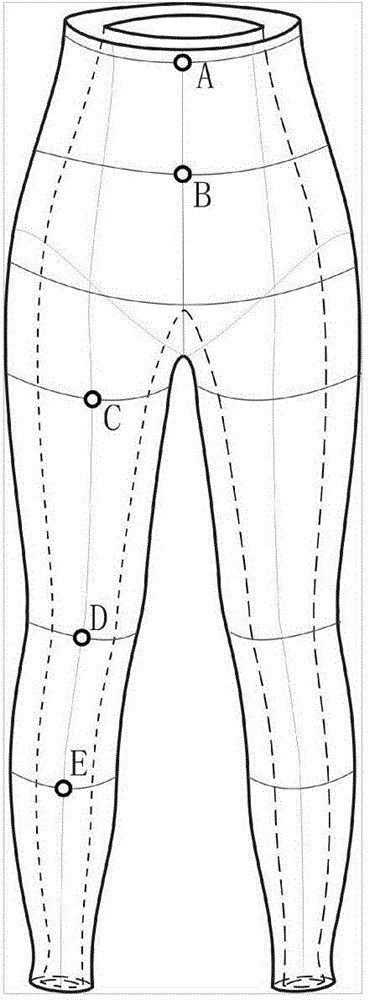
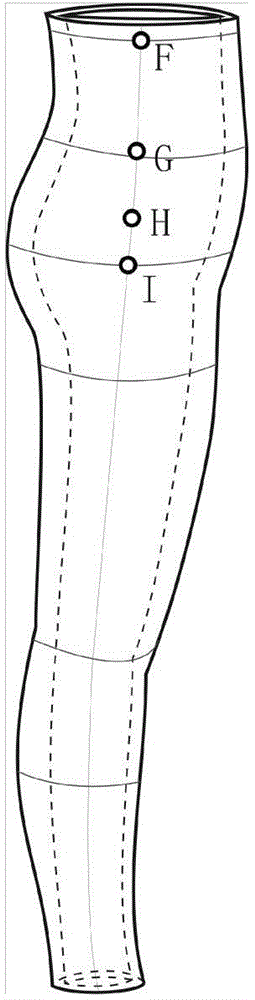
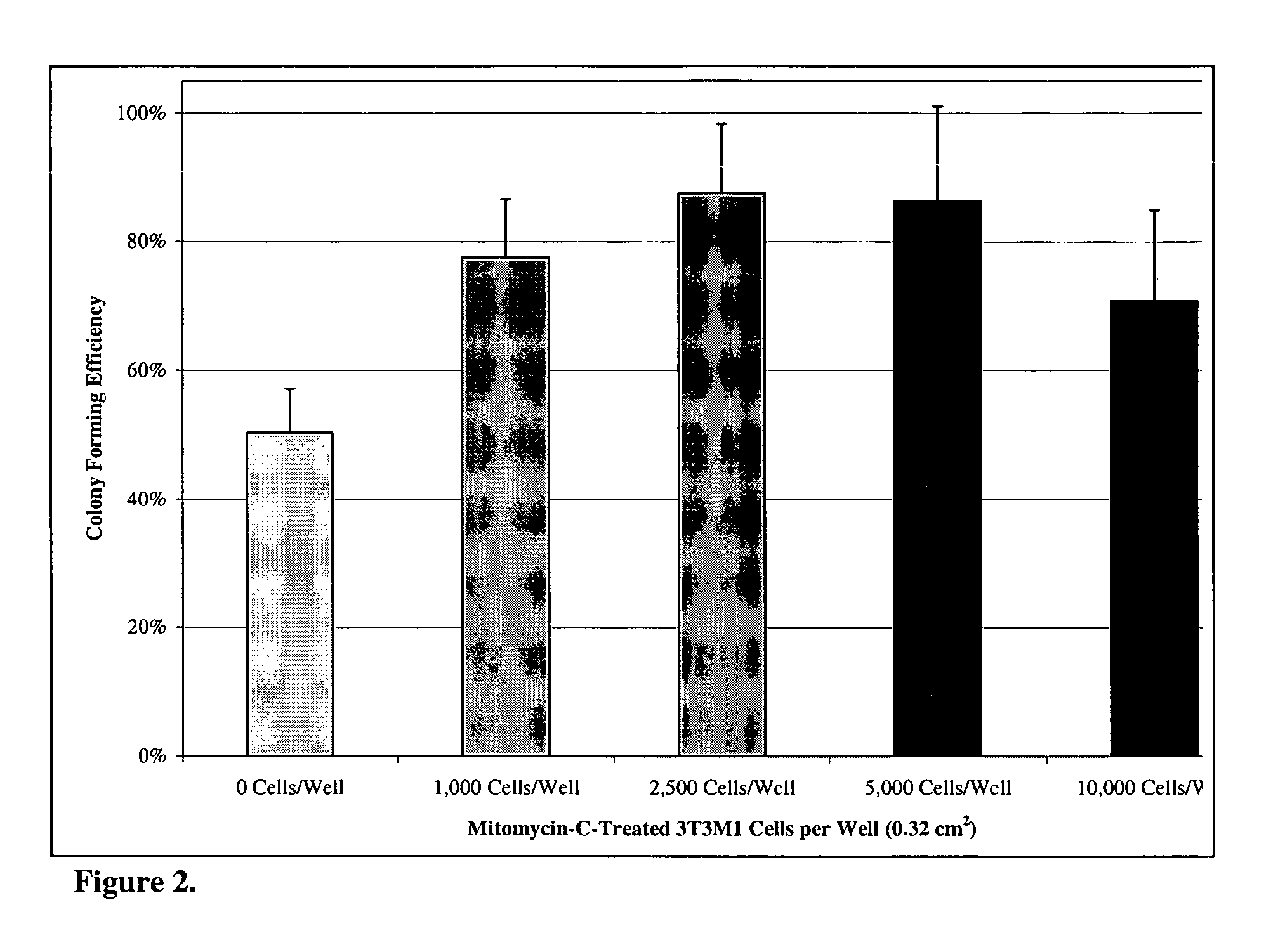
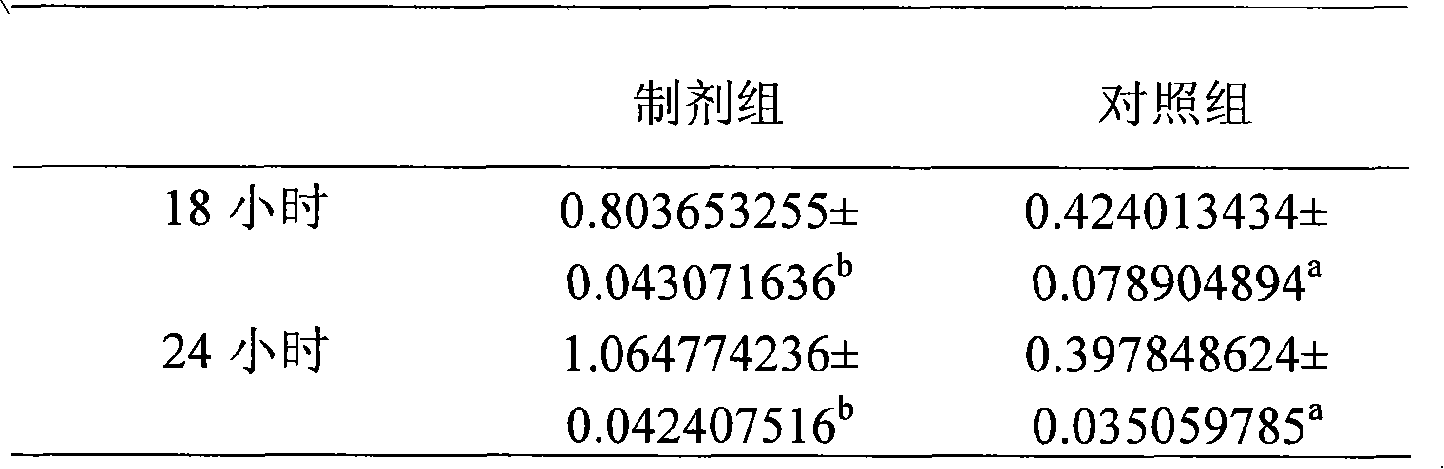
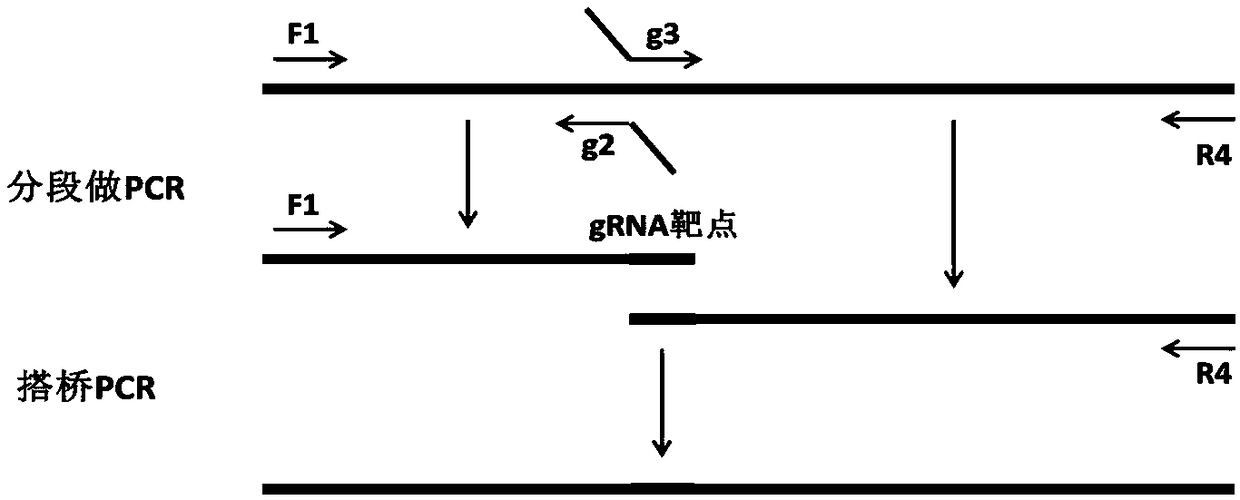
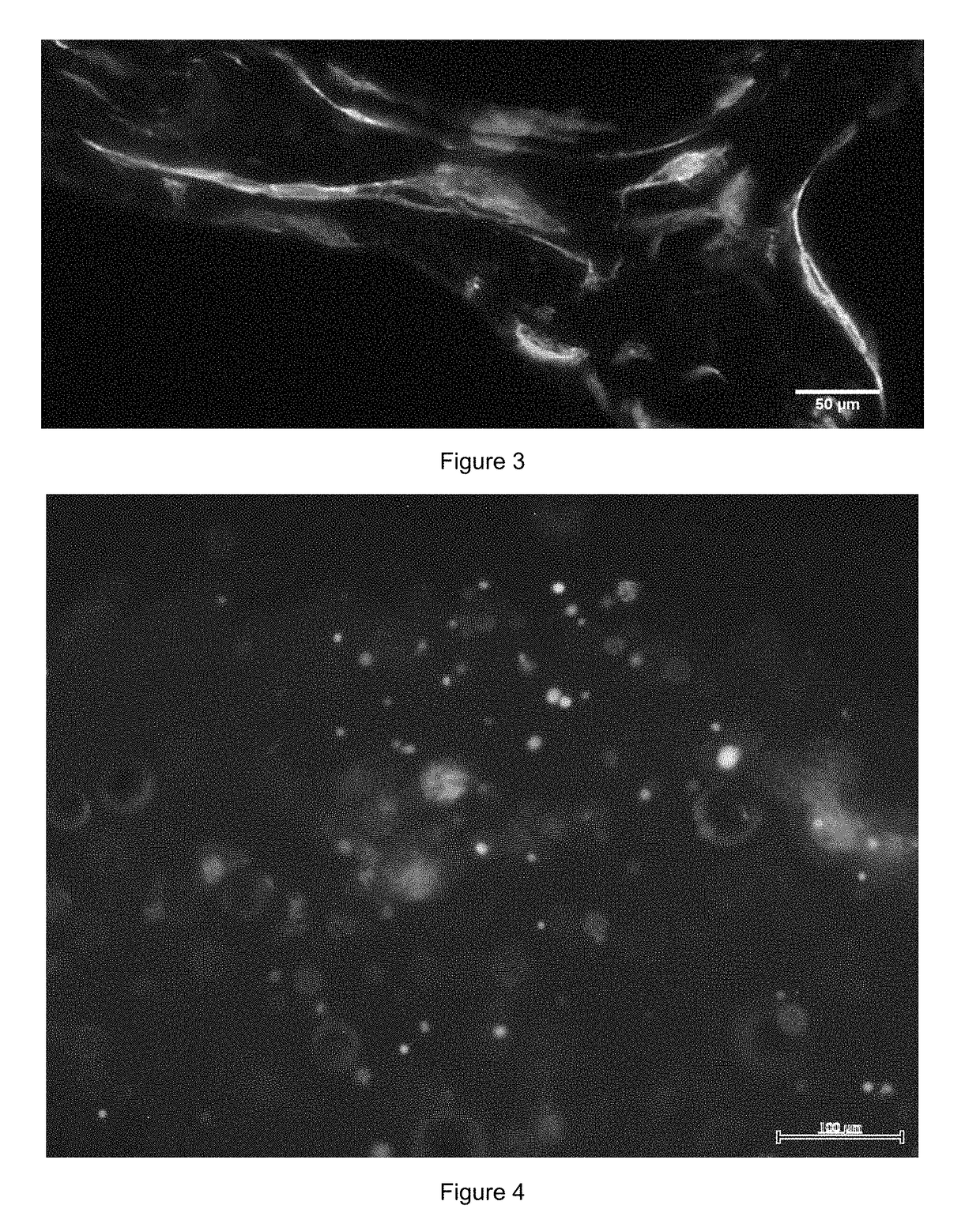
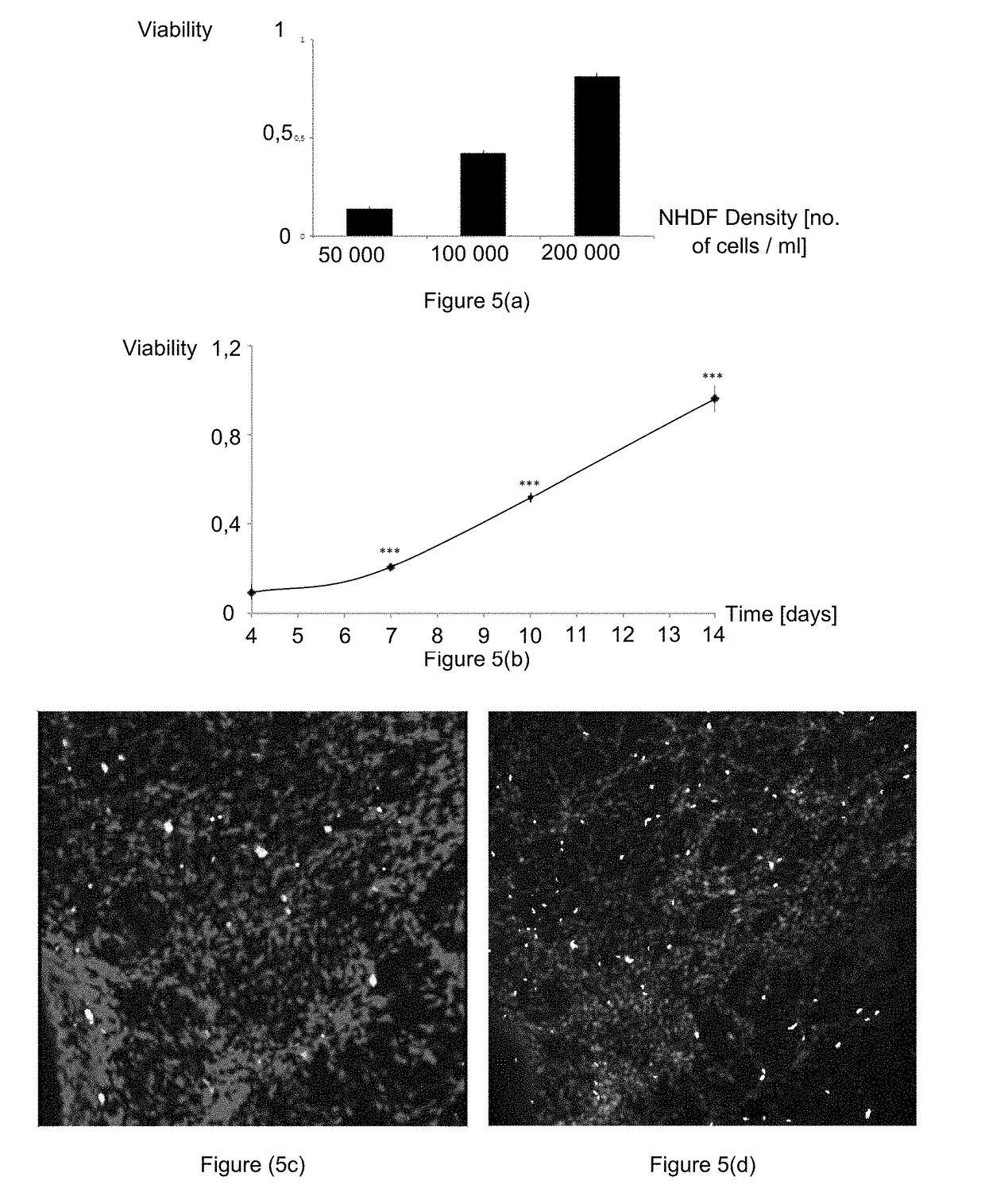
Patents
Literature
95 results about "Skin substitutes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
These skin substitutes include: Epicel: Also known as cultured epithelial autograft (CEA), it only provides the epithelial (outermost) layer of skin. Alloderm: This permanent substitute consists of a treated dermis layer of human cadavers skin, which is mainly used as a dermal implant to replace soft tissue defects.
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
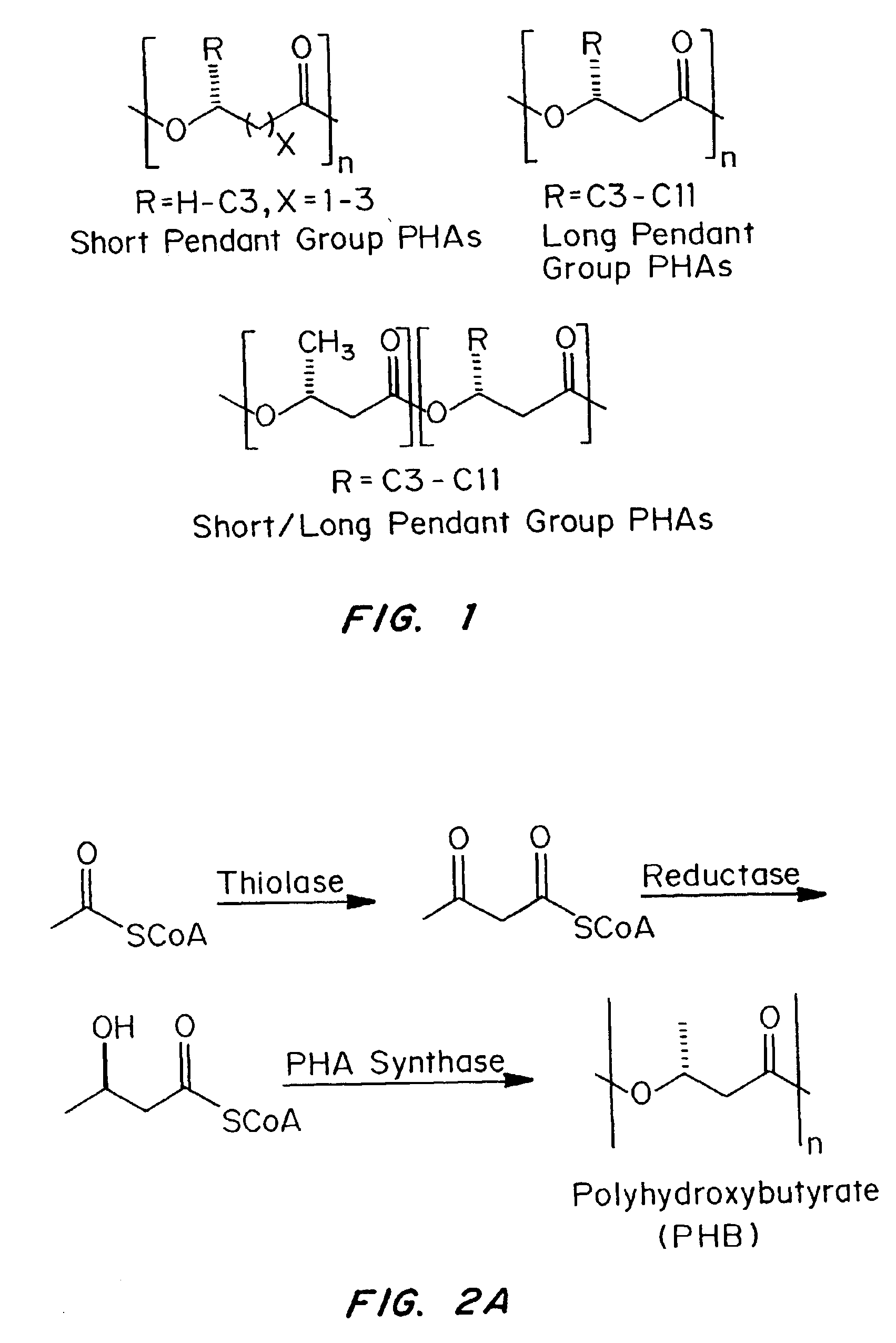
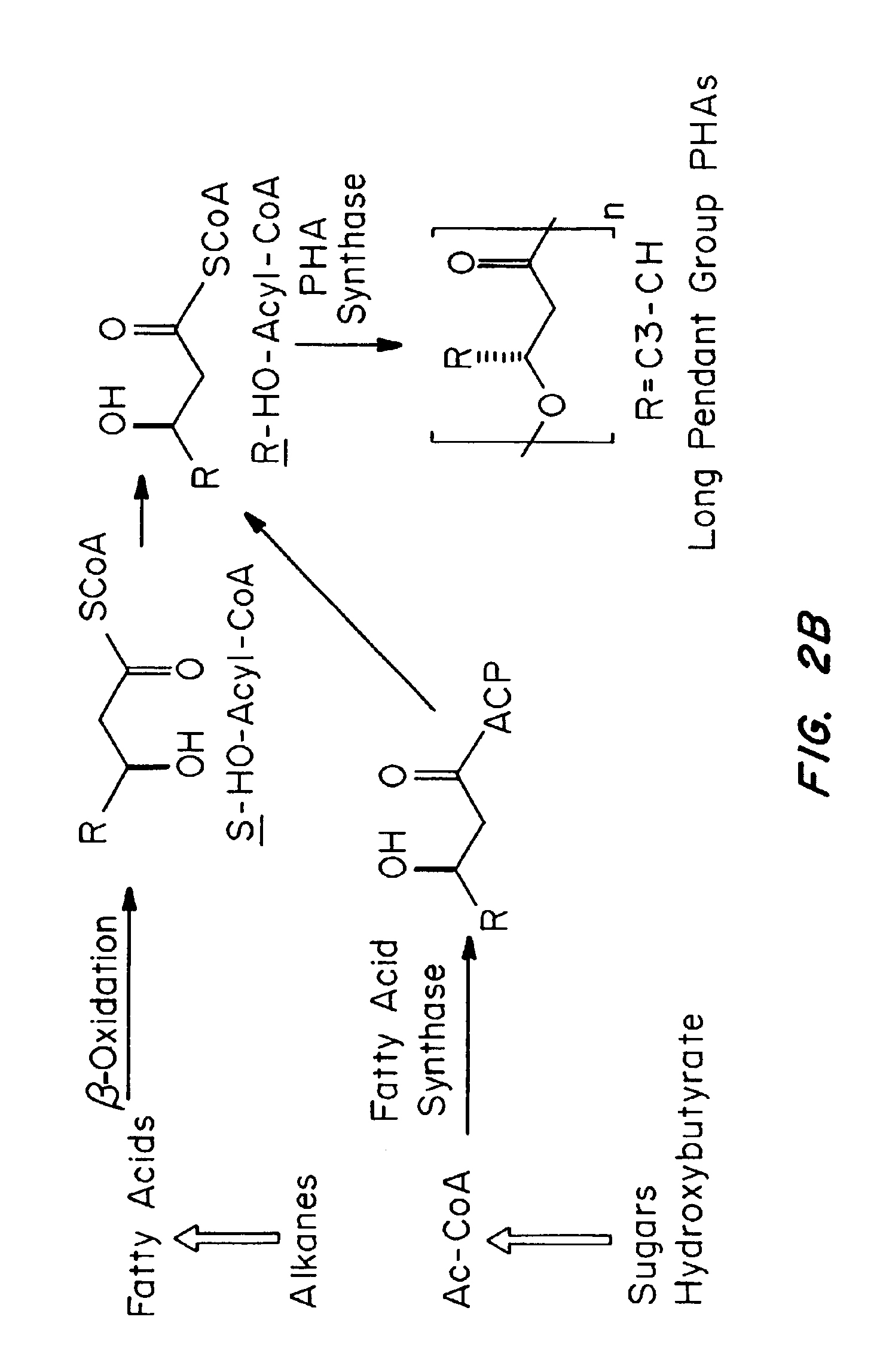
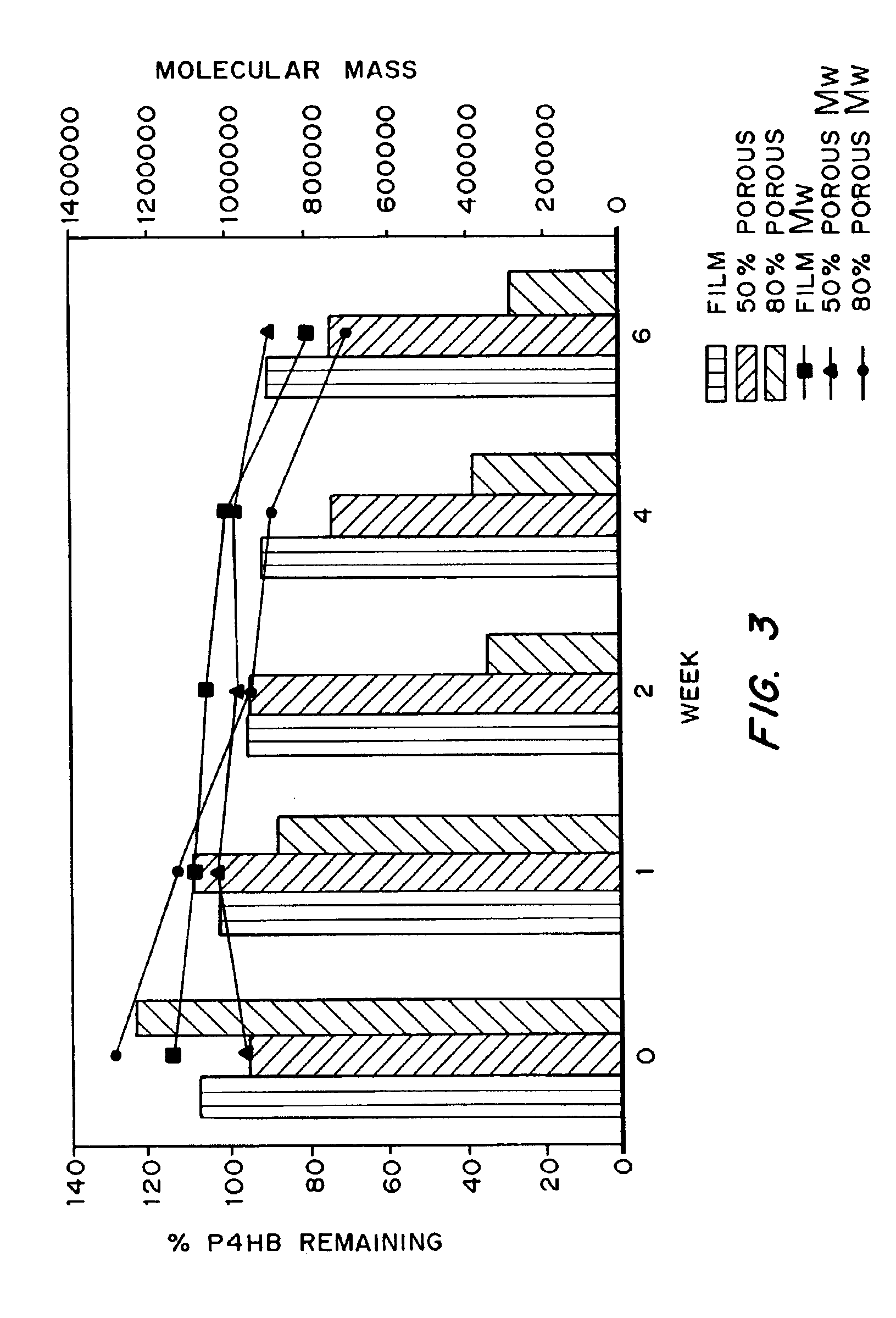
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6867247B2Reduce probabilityHigh porositySuture equipmentsStentsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Skin substitutes and uses thereof
The present invention relates to in vitro cultured skin substitutes, and in particular to improved methods for organotypic culture of skin substitutes. In some embodiments, the dermal equivalent of the skin substitute is lifted to air interface of the culture prior to seeding with keratinocytes. In other embodiments, increased concentrations of collagen are used to form the dermal equivalent. In still other embodiments, optimized media are utilized to maintain the skin equivalents.
Owner:STRATATECH
Skin substitutes with improved barrier function
The present invention relates to in vitro cultured skin substitutes, and in particular to in vitro cultured skin substitutes that have improved barrier function. In some embodiments, improved barrier function is a result of improved culture conditions, while in other embodiments, improved barrier function results from genetic modification of keratinocytes. Improved culture conditions to improve barrier function include organotypic culture in the presence of linoleic acid and / or linoleic acid at about 75% humidity. Suitable genetic modifications for improving barrier function includes transfection with a DNA construct capable of expressing GKLF.
Owner:STRATATECH
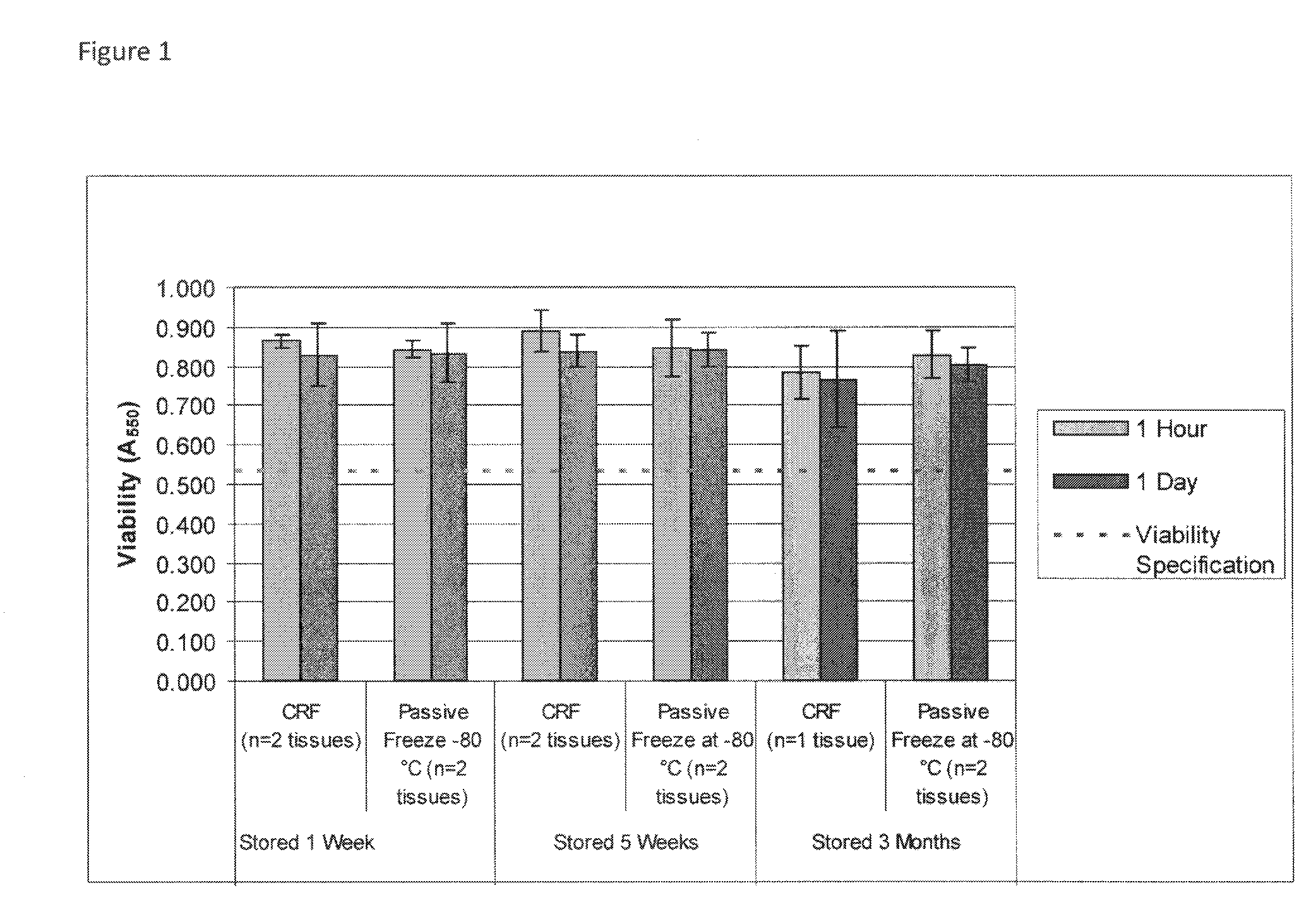
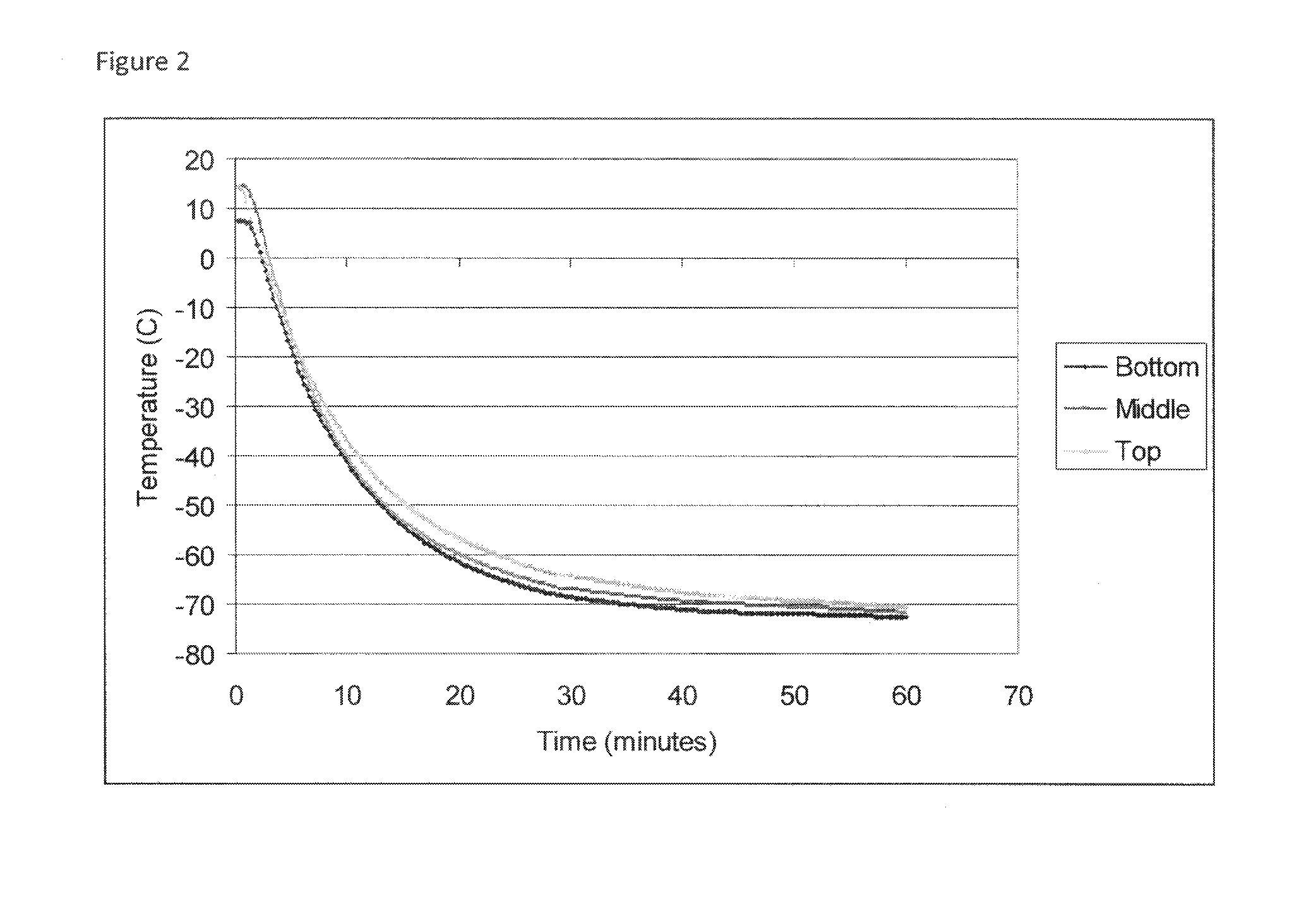
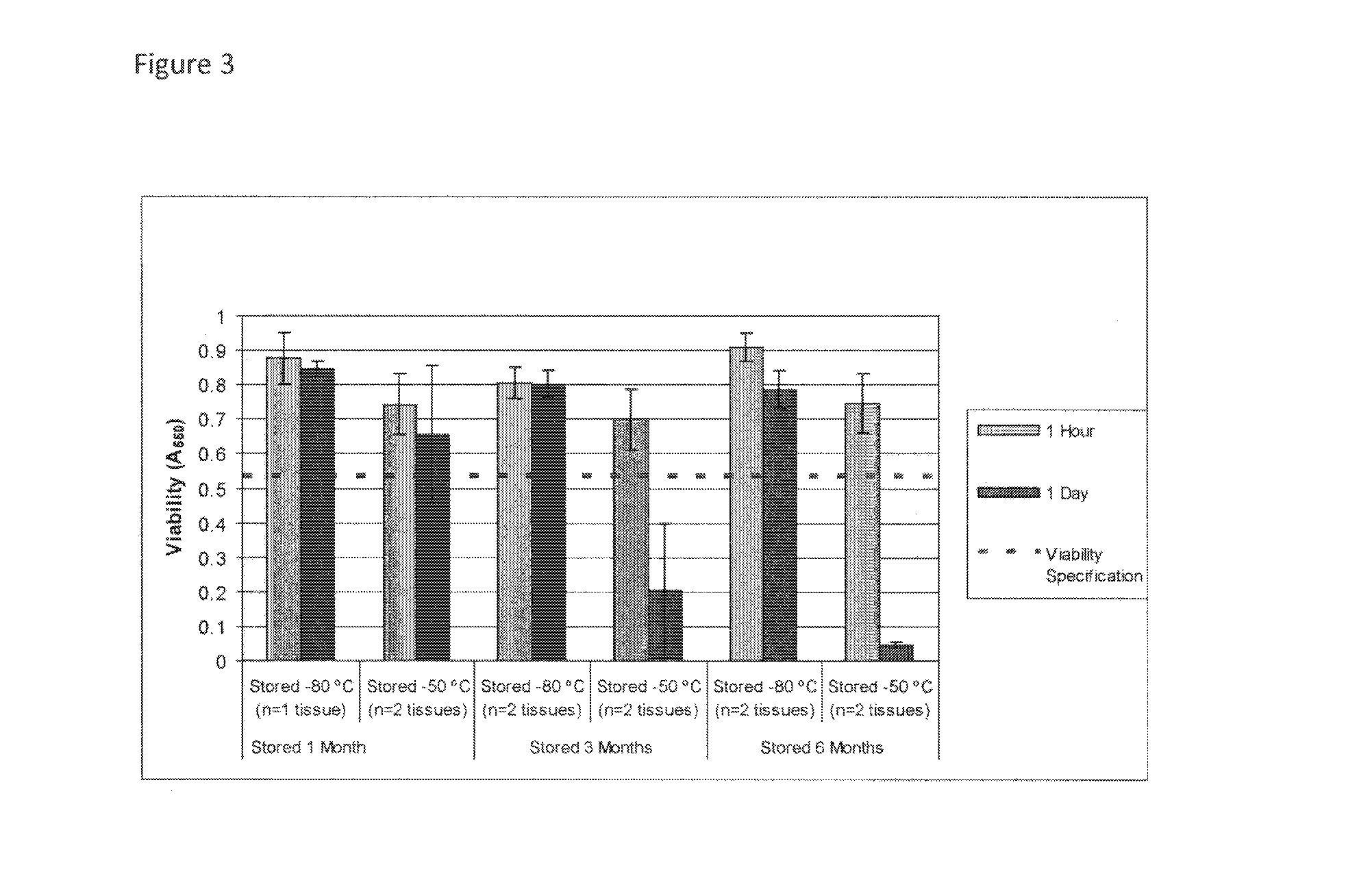
Cryopreservation of viable human skin substitutes
ActiveUS20140271583A1Simple methodBiocideGenetic material ingredientsSkin equivalentCryopreservation
Owner:STRATATECH
Acetobacter xylinum and method for preparing nano-cellulose skin tissue repair material by using the same
InactiveCN101302486AGood biocompatibilityImprove plasticityBacteriaMicroorganism based processesBiocompatibility TestingMoisture
The invention provides an Acetobacter xylinum Y05 strain and a method for preparing nanometer cellulose skin tissue repairing material with the same. The preparation method comprises the following steps that: Acetobacter xylinum Y05 (CCTCC M 207163) preserved on an inclined plane is made into a shake-flask seed and cultivated statically to obtain a nanometer cellulose membrane; and the well-cultivated membrane is separated, purified and then compounded with natural polysaccharide (chitosan, etc.) and protein (collagen, silk fibroin, etc.), so as to prepare the repairing material. The nanometer repairing material has good biocompatibility, flexibility and strength which are similar to human skin, as well as good plasticity and elasticity, can be suitable for various irregular wound surfaces, can keep moisture for a long time, and can be used as a skin substitute and medical dressings for burns, chronic skin ulcers and other skin injuries or defects in clinical practice.
Owner:HUAZHONG UNIV OF SCI & TECH
Cell-less composite type artificial skin and preparation thereof
The invention relates to a cell-free compound artificial skin and a preparation method thereof. The compound skin of the invention is provided with a double layer structure of a dermis layer and an epidermis layer; the dermis layer is a loose porous structure made of extracellular matrix; gel-shaped epidermis-like materials are coated on the upper surface of the dermis layer to solidify, thus forming the epidermis layer, and the epidermis layer is inserted into a void structure of the surface of the dermis layer; compared with the existing skin substitutes, the skin of the invention has the functions of promoting the regeneration of wound skin, enhancing the elasticity, flexibility and mechanical wear resistance of the skin after the wound is healed, reducing the scar proliferation, controlling the contracture, improving the success rate of skin implantation and improving the healing quality; the skin of the invention has a sustained release function and can make individual-based treatment for different diseases by releasing different drugs and being directly applied to the treatment of skin defects caused by inflammations, ulcers, burn wounds and iatrogenic causes; the epidermis layer and the dermis layer are embedded together organically with a tight structure; the shapes, sizes and the thicknesses of the skin can be prepared according to specific requirements. The skin of the invention has wide sources of preparation materials, simple production process, short production period, long period of product storage and convenient transportation.
Owner:西安组织工程工程技术研究中心
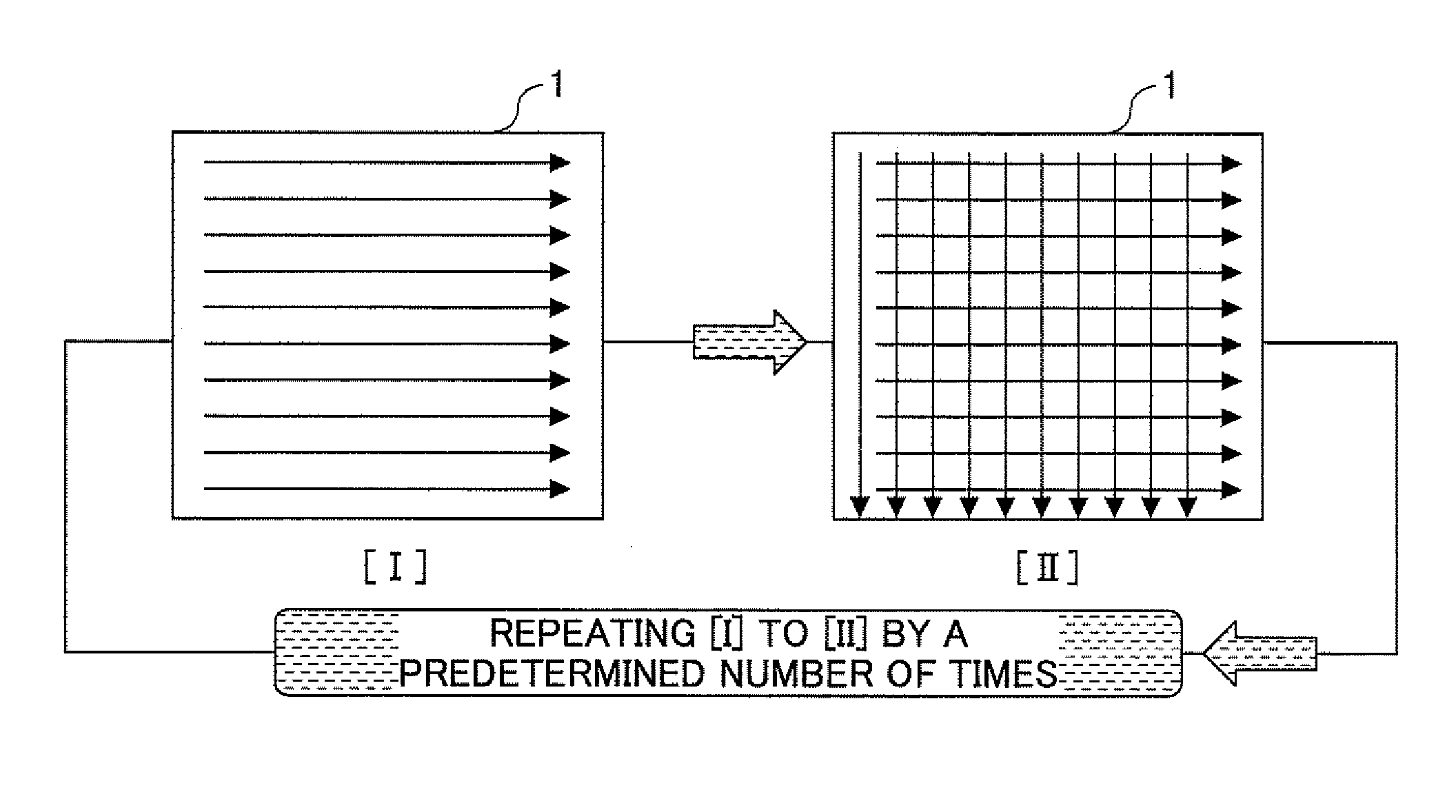
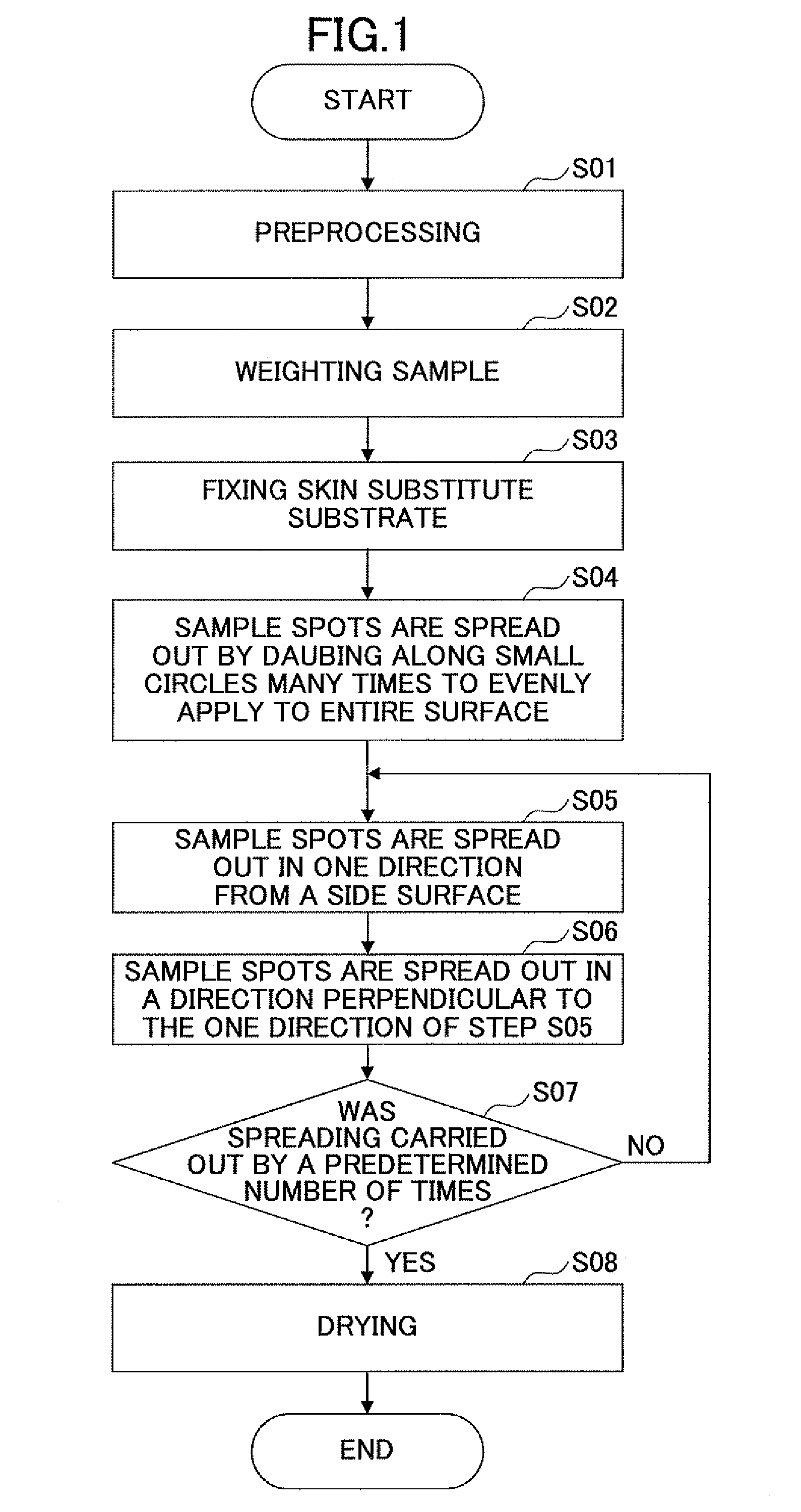
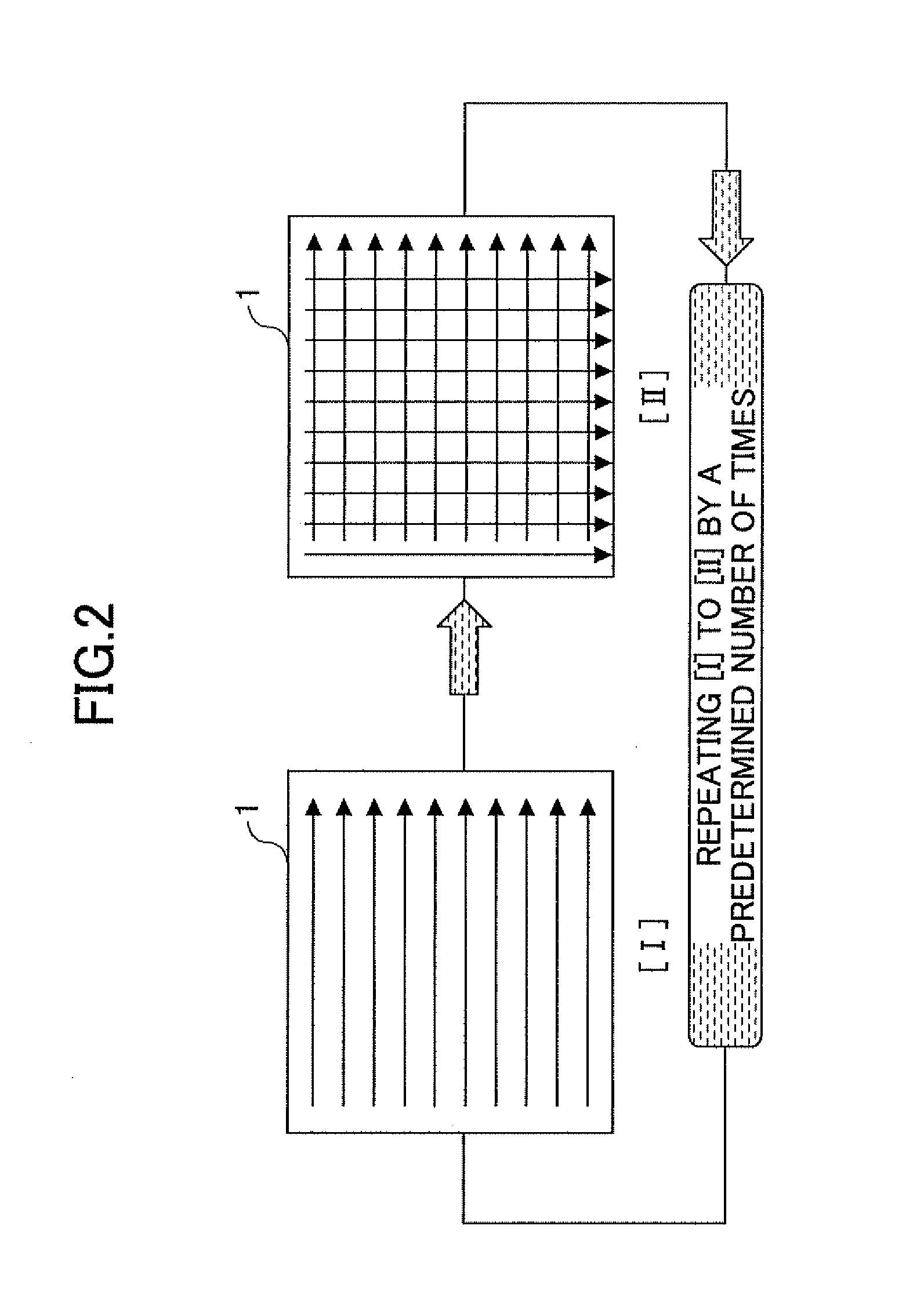
Application method of external dermatological medications, evaluating method of the same, application evaluating apparatus, and application evaluating program
InactiveUS20120022472A1Good repeatabilityCosmetic preparationsDiagnostics using lightDermatologySkin substitutes
A method of applying an external dermatological medication to a skin or a skin substitute substrate with an external dermatological medication for evaluating characteristics of the external dermatological medication acquired by irradiating the skin or the skin substitute substrate with light including a first applying step of spreading the external dermatological medication in one direction on the skin or the skin substitute substrate from a side surface of the skin or the skin substitute substrate; a second applying step of spreading the external dermatological medication in a direction perpendicular to the one direction on the skin or the skin substitute substrate, wherein the first applying step and the second applying step are repeated by a predetermined number of times.
Owner:SHISEIDO CO LTD
Tissue engineered skin with basilar membrane and construction method thereof
InactiveCN101954124ASimple methodEasy to get materialsArtificial cell constructsVertebrate cellsEpidermis structureManufacturing technology
The invention relates to the technical fields of tissue engineering and medical wound repair. At present, a living skin substitute constructed by using the materials of polylactic acid, polyglycolic acid, collagen, hyaluronic acid, and the like as a dermic bracket has the defects that on one hand, host materials are difficult to extract, the living skin substitute has complicated manufacturing technology and is expensive in cost and difficult to widely popularize and apply clinically; and on the other hand, the living skin substitute does not have a skin basilar membrane structure so that healed skin does not resist pressure and wear, and the living skin has unfirm adhesion to the epidermis and is easy to shed and break or form water blisters so that the structural and morphological development of the normal epidermis is influenced; and allogeneic acellular dermis is taken from cadaver skin and is limited in sources and expensive in cost, and thus clinical application is limited. The invention aims at providing a skin substitute which uses surface-finished and modified amnion as the basilar membrane and blood plasma as stroma, which has the advantages of wide material sources, low cost and simple preparation method. An animal experiment proves that the complete basilar membrane and hemidesmosomes can be retained in in-vivo transplantation, and the formation of an epidermal structural form is accelerated and promoted.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Support material for tissue engineering, for producing implants or implant materials, and an implant produced with the support material
InactiveUS7763272B2High fiber densityStable against compressionAdditive manufacturing apparatusSkin implantsIn vivoColonization
A scaffold for tissue culture and cell culture and for producing implant materials, in particular bone, cartilage or skin replacements or extra-corporal organ replacements or for other applications in medicine or biotechnology is made of biocompatible materials. It has at least one base material which is electrostatically flocked with fibers on at least one side. Through the electrostatic flocking the fibers are arranged almost perpendicularly on the surface of the base material and exhibits a high fiber pull-out resistance. The scaffold provides an elastic growth lattice, which is stable against compression, for cell colonization in vitro or the ingrowth of cells in vivo. Implants or implant materials can be produced with the scaffold.
Owner:DRESDEN UNIVERSITY OF TECHNOLOGY
Production method of embedded-sensor simulation software dummy for clothing pressure test
InactiveCN104392063AAccurately reflect the mechanical propertiesReflect mechanical propertiesSpecial data processing applicationsHuman bodySilica gel
The invention relates to a production method of a embedded-sensor simulation software dummy for a clothing pressure test. The production method includes regarding real body data, in a certain sample size, of humans of a certain age as samples, and acquiring intermediate body forms and sizes of corresponding parts by means of mathematical statistics; selecting a model close to the intermediate body in the sizes of height, waistline, hipline, abdominal perimeter and the like, extracting a three-dimensional human body model, and producing a three-dimensional human body model form of the intermediate body by the reverse engineering; according to human soft tissue thickness, producing a three-dimensional model of a hard inner shell of the model form by narrowing inwards the intermediate body; printing the hard inner shell of the model form by the rapid proto-typing technology, with a model form soft tissue made of human body silicon, and human body skin substitute material which is smooth elastic fabric; selecting sensor embedding points, fixing sensors onto the surface of the human body soft material, allowing guide lines to penetrate the soft material and the hard inner shell to be connected with an outside data collecting device outside the body. The production method is convenient and effective to use to evaluate body pressure comfort.
Owner:DONGHUA UNIV
Multifunctional Compound Skin Or Wound Dressing As Regenerative Skin Substitute
Provided is a new bionic skin material with multiple functions and a high efficacy. With a compound microporous fibre net structure, the material imitates a skin barrier function, has anti-fouling properties, hygroscopic properties, moisture retention properties, air permeability, flexibility and adhesiveness, and can effectively absorb wound exudate and residues for rapid painless autolysis and debridement, ensure sufficient drainage by bidirectional regulation, maintain a semi-closed, local physiological wet environment favorable for the reparative metabolism and physiological healing of a wound, inhibit inflammation, reduce exudation, benefit the response inside and outside of cells, promote the migration of fibroblasts and epithelial cells of the body itself and the regeneration of granulation tissue and epithelial cells of the wound, accelerate the physiological healing and recovery of the wound, and reduce recurrences. It has been clinically proved that the dressing material can accelerate the healing of acute skin injuries and chronic ulcer wounds, achieve a high healing quality, avoid or reduce traumatic skin grafting to the greatest extent, reduce the pain of patients and the production of scars, and restore the physiological function and appearance of the body.
Owner:杭州弘复医疗科技有限公司
Human skin substitutes expressing il-12
InactiveUS20100330046A1Prevents and inhibits spreadBiocidePeptide/protein ingredientsHuman skinCancer research
The present invention relates generally to compositions for treating patients that have skin cancer or have recently had skin cancers removed. More specifically, the present invention provides human skin substitutes engineered to express exogenous IL-12 and compositions and methods for making human skin substitutes engineered to express exogenous IL-12. In addition, the present invention provides methods for treatment of sites on a patient where skin cancers have been removed with human skin substitutes engineered to express exogenous IL-12.
Owner:STRATATECH
Double-layer tissue engineering skin and preparation method thereof
The invention discloses a tissue engineering double-layer skin and a preparation method thereof. The tissue engineering double-layer skin is characterized by being constructed by using fibroblast cells and epidermal cells as seed cells and an acellular amniotic membrane as a biological scaffold. The tissue engineering double-layer skin disclosed by the invention has the advantages of low cost, simple operation, wide sources and easy storage, and is a skin substitution having high transplantation efficiency. The invention also provides the preparation method of the tissue engineering double-layer skin.
Owner:GUANGZHOU RAINHOME PHARM&TECH CO LTD
Skin substitutes and uses thereof
The present invention relates to in vitro cultured skin substitutes, and in particular to improved methods for organotypic culture of skin substitutes. In some embodiments, the dermal equivalent of the skin substitute is lifted to air interface of the culture prior to seeding with keratinocytes. In other embodiments, increased concentrations of collagen are used to form the dermal equivalent. In still other embodiments, optimized media are utilized to maintain the skin equivalents.
Owner:STRATATECH
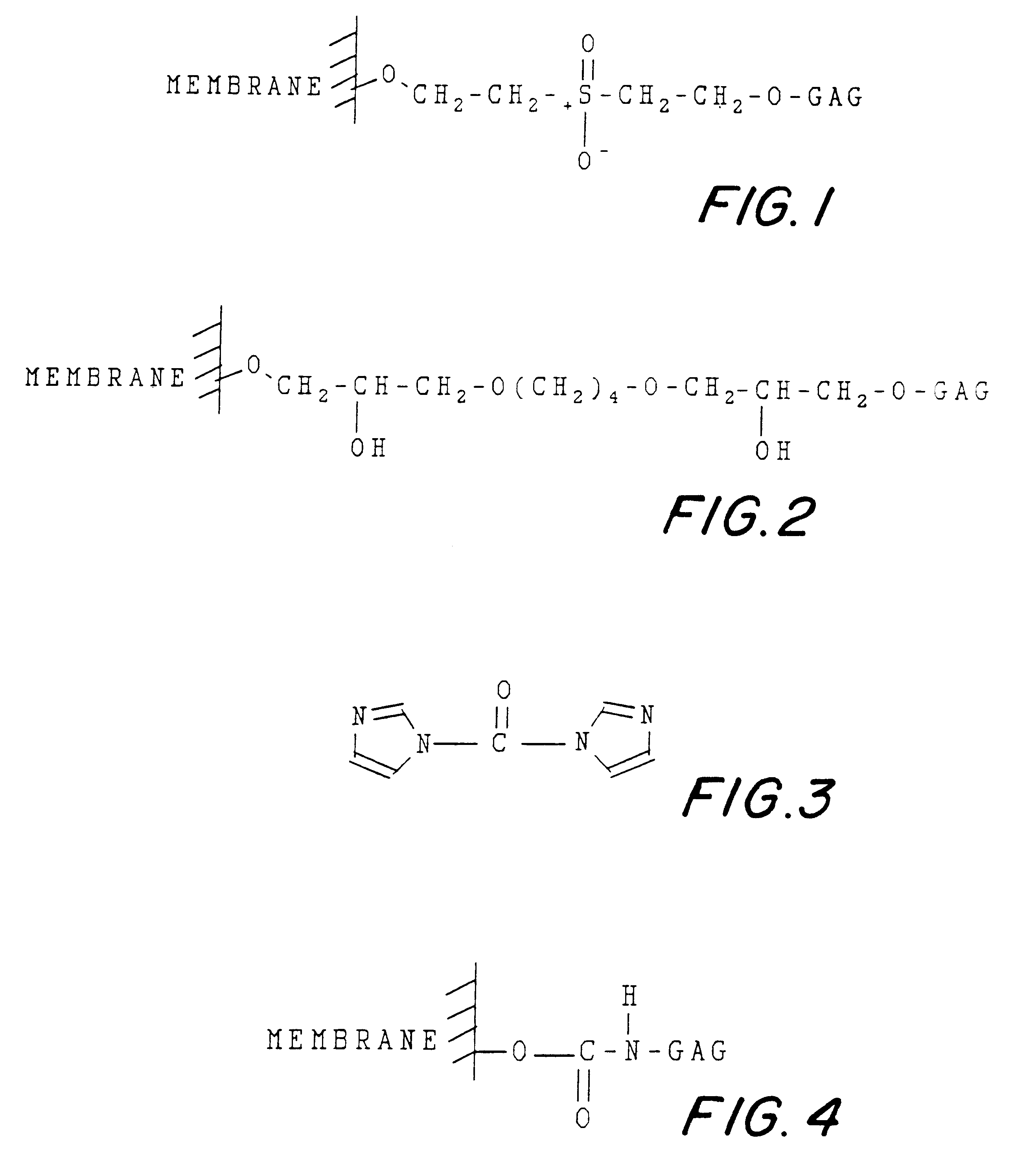
Non-immunogenic, biocompatible macromolecular membrane compositions, and methods for making them
InactiveUS6262255B1Minimizing antigenic contaminantSure easyOrganic active ingredientsPowder deliveryCellulose(Hydroxyethyl)methacrylate
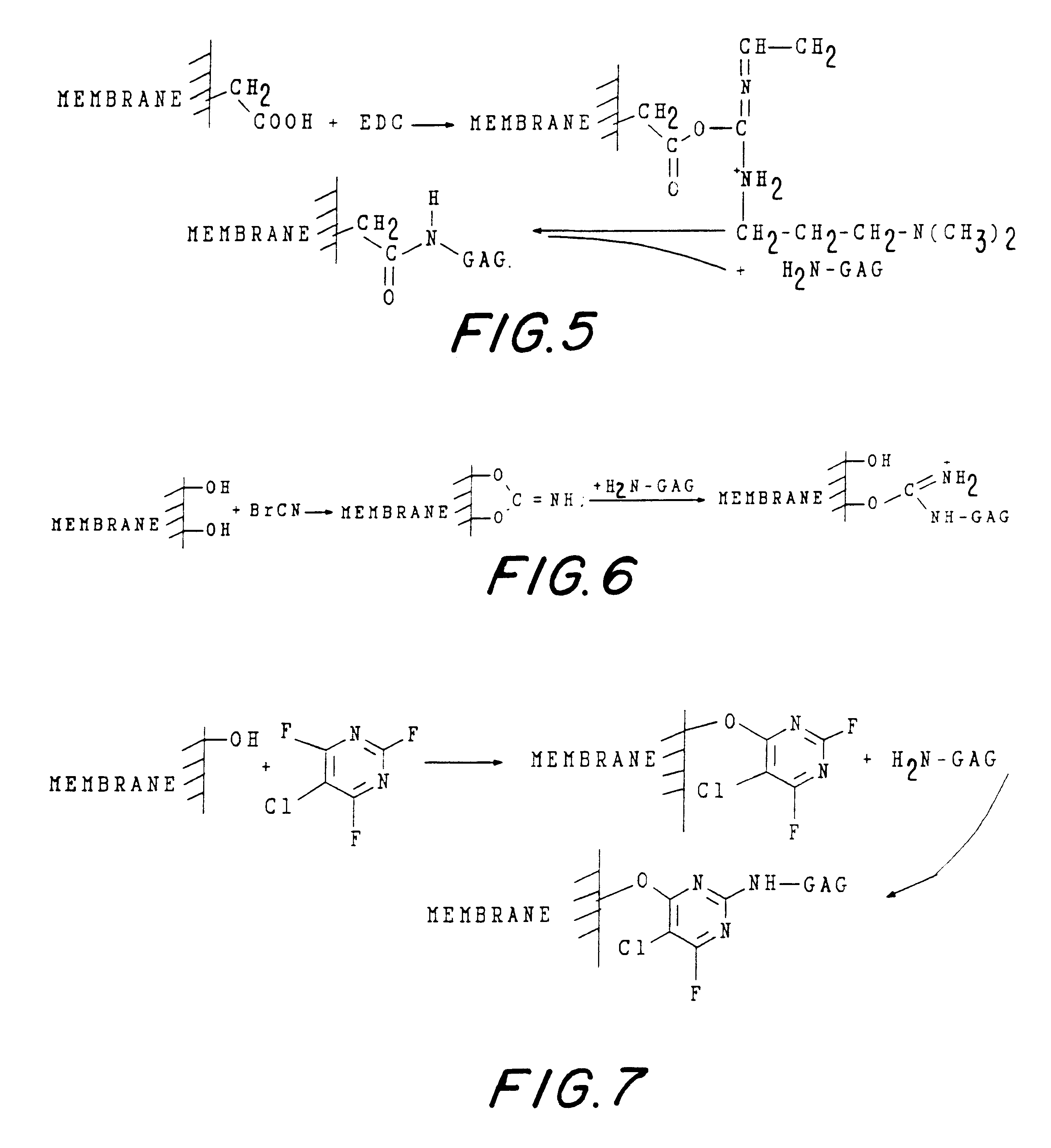
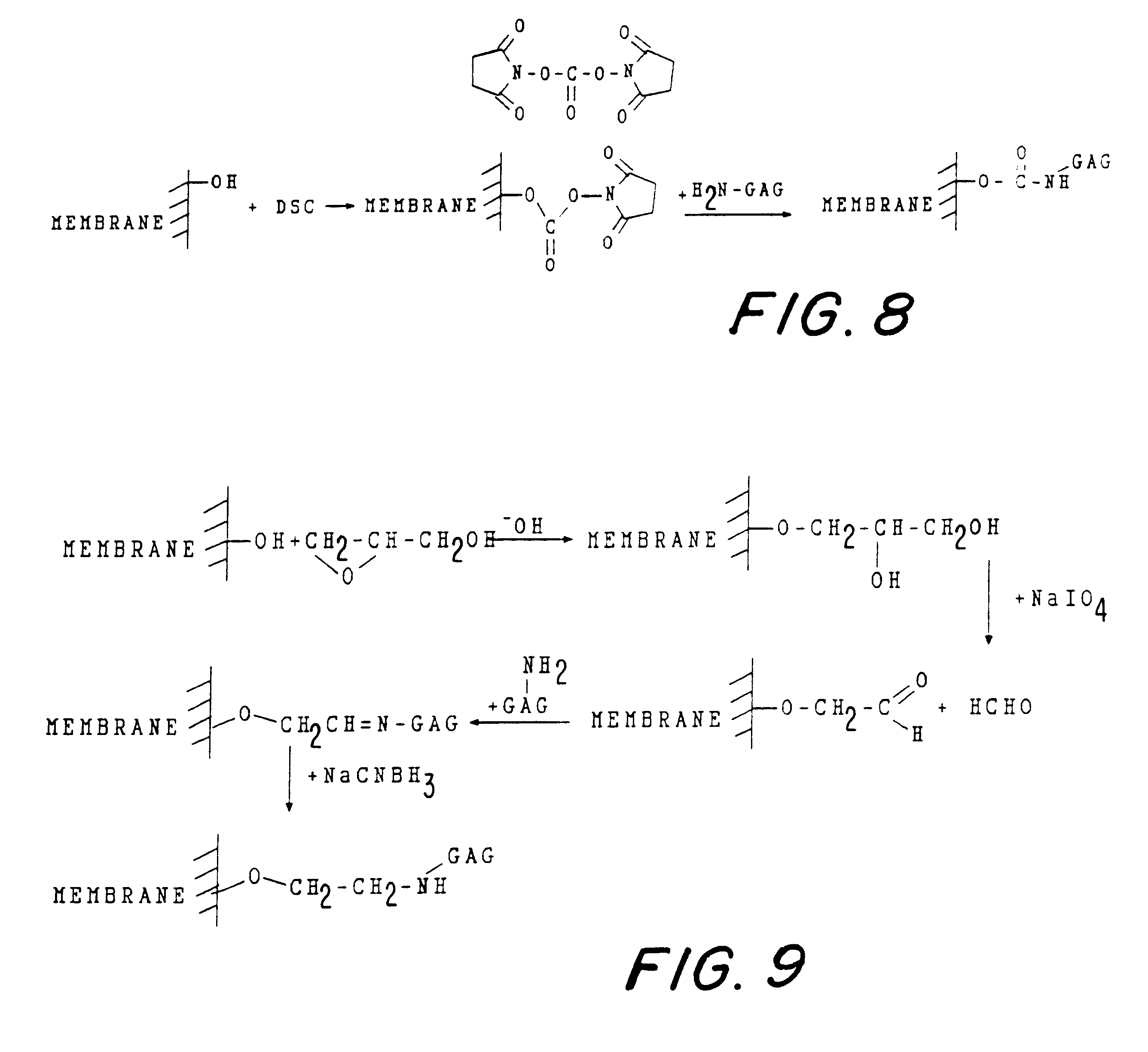
Non-immunogenic biocompatible macromolecular sheet composition are formed from a cellulosic membrane, a binding moiety having a plurality of functional groups, and a glycosaminoglycan (GAG). The binding moiety has the formula of R1-X-R2 wherein R1 and R2 are the same or different. The binding moiety, through functional groups binds the cellulosic membrane with the glycosaminoglycan. R1 is covalently bound to a carbon or an oxygen of the cellulosic membrane. R2 is covalently bound to a carbon, an oxygen, or a nitrogen of the glycosaminoglycan. The binding moiety can be bis-oxyrane, butanediol-diglycidyl ether (BDE), or divinyl sulfone. The cellulosic membrane can be a cellulosic membrane, partially acetylated cellulose and a copolymer of hydroxyethyl-methacrylate with methyl methacrylate, abbreviated as HEMA-MMA. The non-immunogenic biocompatible macromolecular sheet composition can be formed into a pouch to encapsulate cells, tissues, pharmaceuticals, or biological metabolic products. In addition, the non-immunogenic biocompatible macromolecular sheet composition can also be used as a skin graft or a skin substitute. Further, these non-immunogenic biocompatible macromolecular sheet composition can also be used as a surface to culture cells in vitro.
Owner:BIOMM
Application of selective cell-removed pork skin as skin substitute of human body and its preparation method
The present invention discloses a new application of pig skin which can be substituted for human skin and its preparation method. Said preparation method includes the following steps: killing health living while pig with about 50 kg, shaving hair and cleaning pigskin, skinning and chipping to obtain skin sheet, cross-linking with glutaraldehyde, cleaning with balanced salt solution phosphate, soaking it in IM physiological saline containing trypsase, vibrating with Triton-100, more washing it with balanced salt solution phosphate and storing it in refrigerator with 4 deg.C for stand-by. Said invented product has the functional characteristics of two skin substitutes of Integra artificial skin and ADM, so that it not only can be used as temporary wound surface covering material for protecting surface of wound, but also can be used as permanent substitute for dermis for repairing surface of the wound.
Owner:陕西艾尔肤组织工程有限公司
Skin substitutes with improved barrier function
The present invention relates to in vitro cultured skin substitutes, and in particular to in vitro cultured skin substitutes that have improved barrier function. In some embodiments, improved barrier function is a result of improved culture conditions, while in other embodiments, improved barrier function results from genetic modification of keratinocytes. Improved culture conditions to improve barrier function include organotypic culture in the presence of linoleic acid and / or linoleic acid at about 75% humidity. Suitable genetic modifications for improving barrier function includes transfection with a DNA construct capable of expressing GKLF.
Owner:STRATATECH
Process for preparing artificial active skin of skin stem cell collagen sponge film
The preparation process of artificial active skin includes planting separated and purified skin stem cell to both up and down sides of collagen sponge film, in vitro inducing the up skin stem cell to differentiate into the epiderm layers, and in vitro inducing the down skin stem cell to differentiate into the corium tissue, so as to form artificial active skin tissue with epiderm and corium tissue structure. The naked Balb / C mouse experiment shows that the artificial active skin tissue may be used in promoting features that epiderm growth and tissue repairing in the wound. The present invention has the advantages of simple process and low cost, and the artificial active skin of the present invention is one kind of excellent skin substitute.
Owner:戴育成
Preparation for promoting fibroblasts to secrete extracellular matrix components and preparation method thereof
InactiveCN101648010AImprove habitatEasy to useCosmetic preparationsPeptide/protein ingredientsCell-Extracellular MatrixHazardous substance
The invention discloses a preparation for promoting fibroblasts to secrete extracellular matrix components, and a preparation method and application thereof. The preparation comprises the components of A) the egg white of eggs, B) egg white lysozyme, and C) pyruvic acid or pyruvate, can also comprise ovotransferrin and / or glucose, and is obtained by mixing the components uniformly. The preparationcan serve as an externally used paste for skin in application, and can obviously stimulate the secretion of collagen and hyaluronic acid synthase in the fibroblasts of the skin and improve the resiliency and the moisture content of the skin. The preparation is derived from a biologic extractive technique, has no harmful substance residues, safe and high-efficiency use, and is different from the prior method for promoting the secretion of a single fibroblast ECM component; besides, the preparation has multiple effects, wide application range and extensive application prospect, and can be applied in the fields of the preparation of skin substitutes, the preparation of anti-aging skin care products, cosmetics, medical treatment and the like.
Owner:BEIJING ZHONGKE YINUO BIOTECH
Organ-type artificial skin as well as preparation method and application thereof
InactiveCN103893831AHas a physiological functionFor long-term storageSkin implantsEpidermis structureCuticle
The invention relates to organ-type artificial skin and a preparation method thereof. The organ-type artificial skin disclosed by the invention is formed by constructing fibroblasts, a human acellular amniotic membrane or keratinocytes or by constructing fibroblasts, amniotic fluid stem cells, a host material and keratinocytes. The artificial skin disclosed by the invention has dermis and epidermis structures close to a normal skin structure and a protein marker expressing normal skin, and distribution of a cuticle, a stratum spinosum, a basal layer and dermis is consistent with that of normal skin; the artificial skin can serve as a skin substitute, skin temporary covering and an artificial skin model.
Owner:BIOPHARM RES & DEV CENT JINAN
Tissue Culture Chip
ActiveUS20150065588A1Preventing and inhibiting diffusionPrevent leakageCompound screeningBiocideHorizontal axisBiology
A dual chamber bioreactor for producing complex, multilayer tissue, organs, organ parts, and skin replacements has been developed. The bioreactor is modular and incorporates a removable tissue culture cassette. By rotating the dual chamber bioreactor along the horizontal axis, different populations of cells with different growth requirements can be cultured on the different surfaces of the tissue culture cassette that are exposed to different media reservoirs. Culturing different populations of cells on different surfaces of the tissue culture cassette enables the production of multilayer tissue and organs. The tissue culture cassette can contain one or more discrete tissue culture sections.
Owner:AUGUSTA UNIV RES INST INC
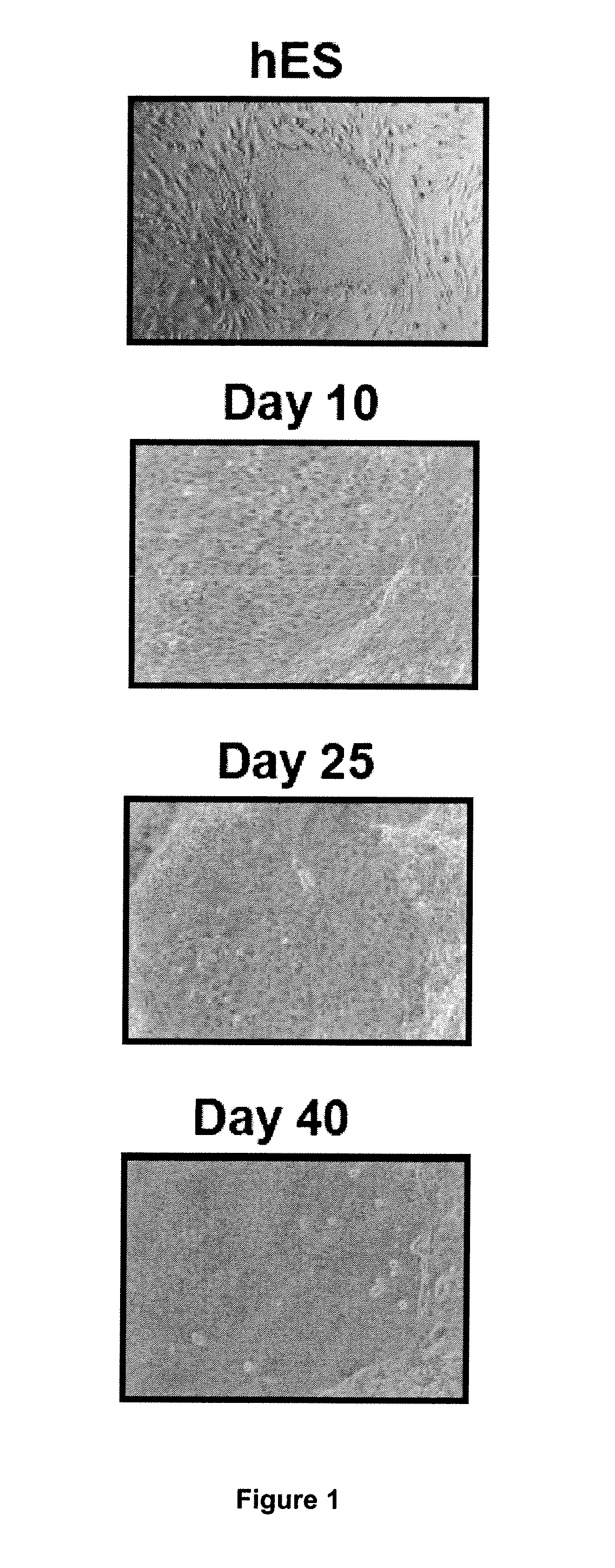
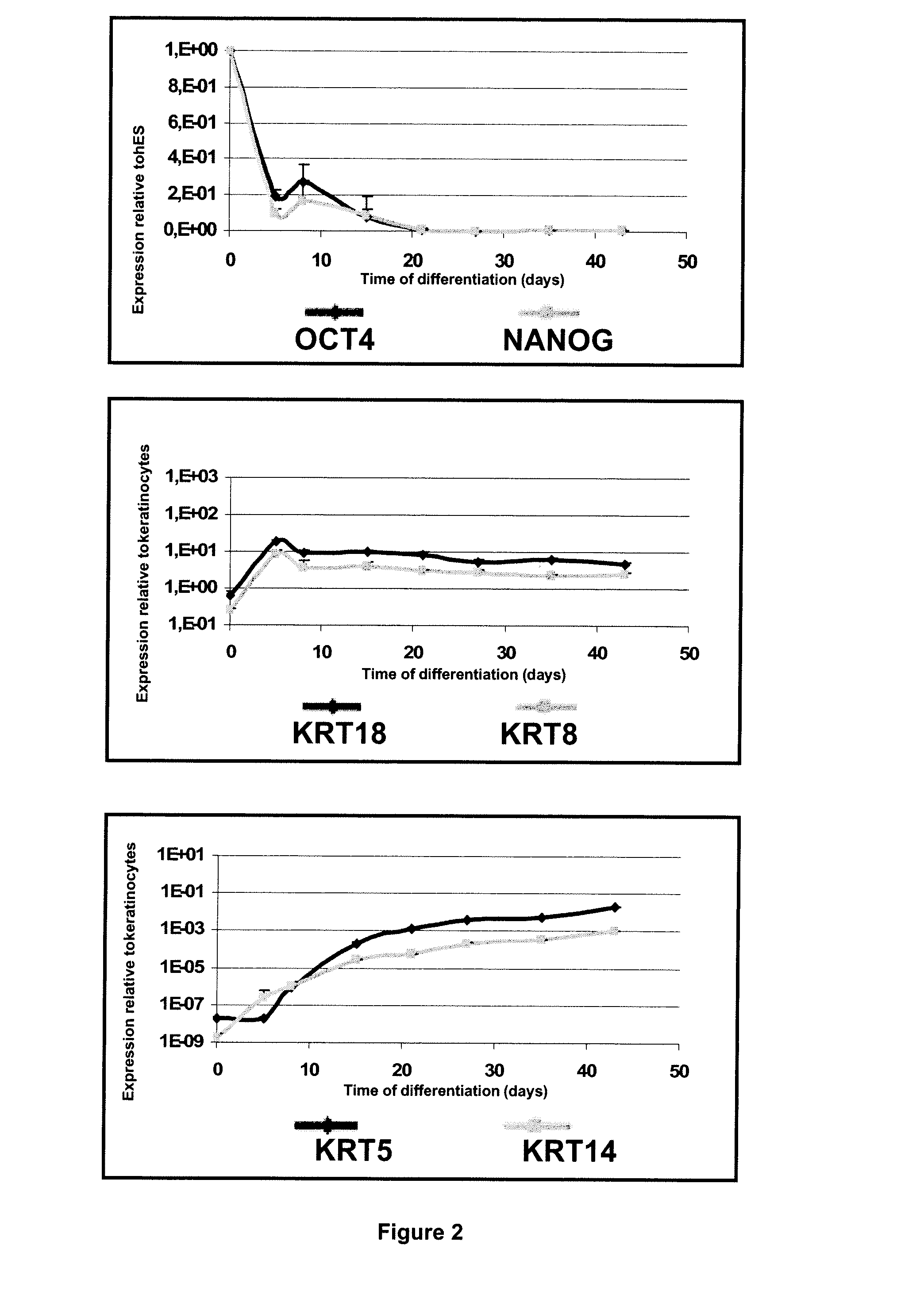
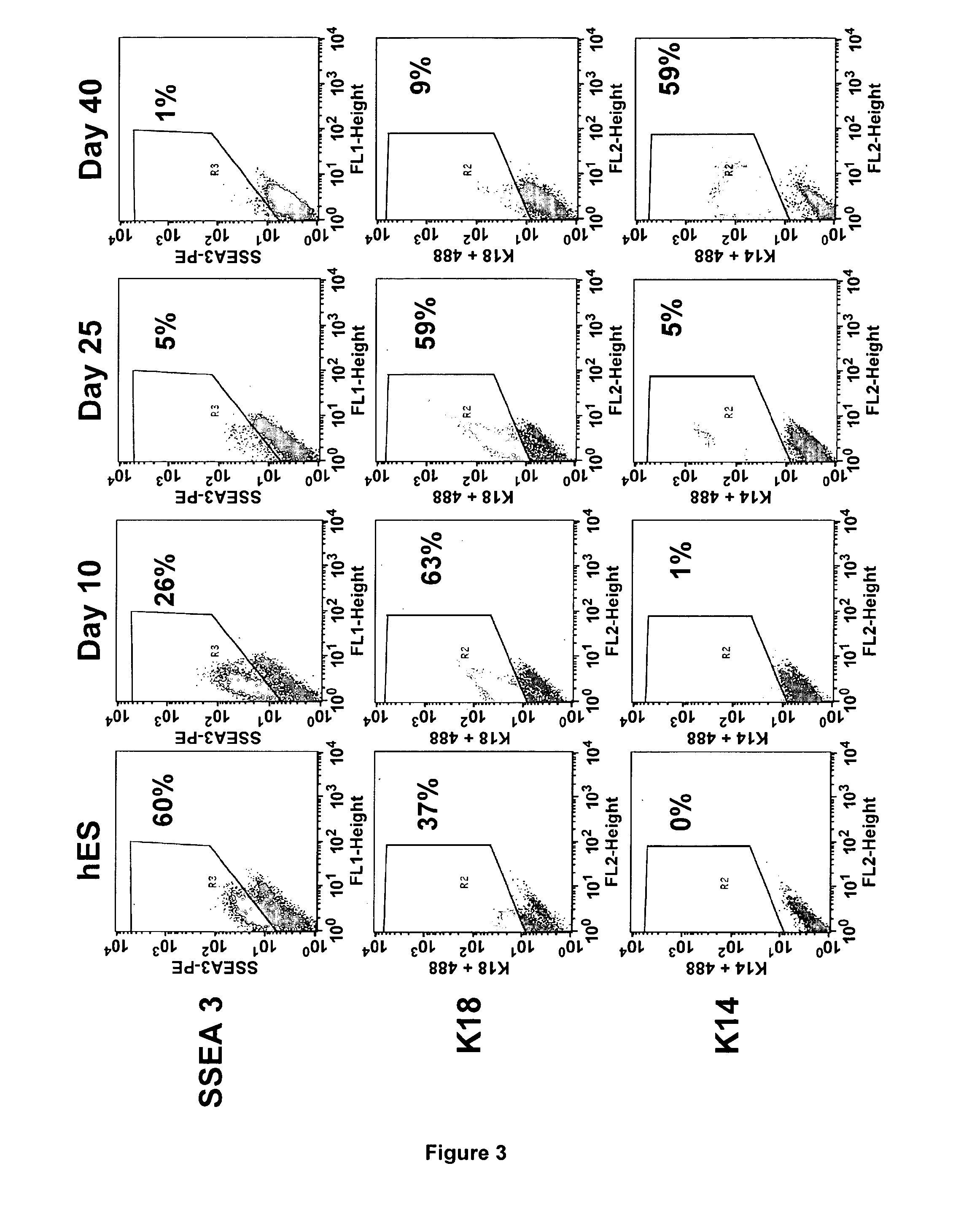
Methods for Preparing Human Skin Substitutes from Human Pluripotent Stem Cells
InactiveUS20110165130A1Enhance stratification and differentiationBiocideEpidermal cells/skin cellsGerm layerCuticle
The present invention relates to an ex vivo method for obtaining a population of human keratinocytes derived from human pluripotent stem cells comprising a step of co-culturing human pluripotent stem cells with cells that support ectodermal differentiation in presence of an agent that stimulates epidermal induction and a agent that stimulates terminal differentiation of keratinocytes. A further object of the invention relates to a method for preparing a human skin substitute comprising a step of providing an organotypic culture of the substantially pure homogenous population of human keratinocytes derived from human pluripotent stem cells obtained according to the method of the invention.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
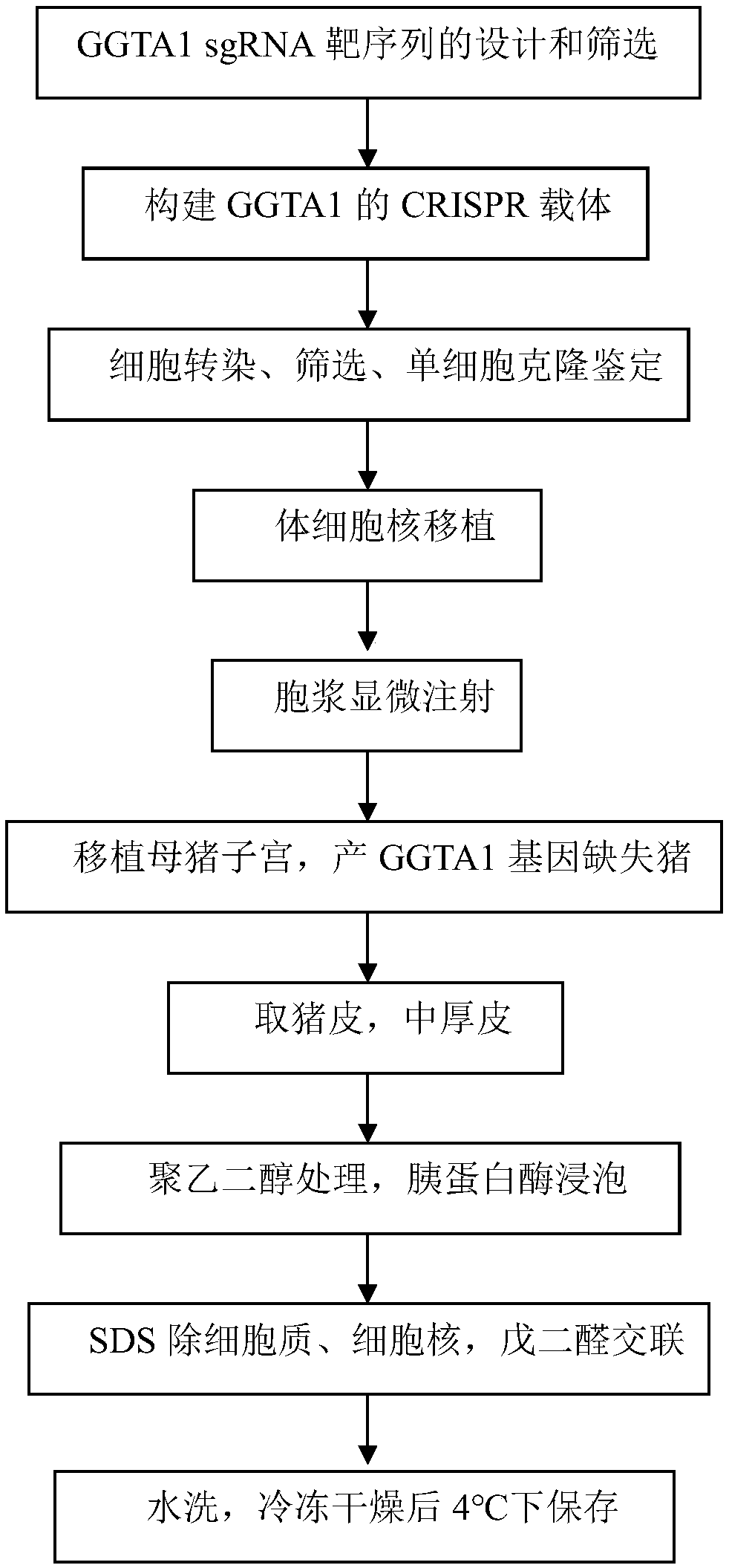
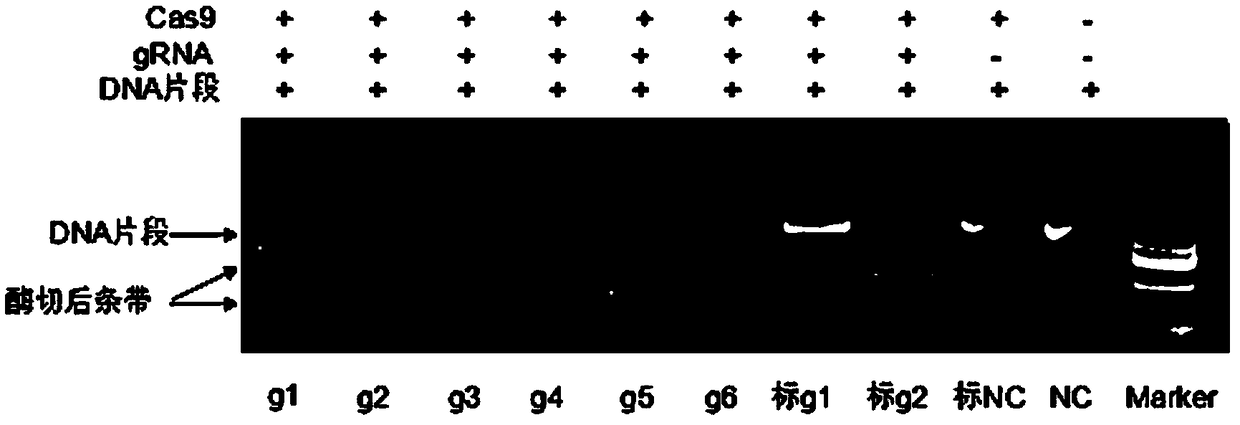
SgRNA for lowering immunogenicity, low-immunogenicity dressing and preparation method thereof
PendingCN108642054ALow immunogenicityNon-immunogenicGenetically modified cellsNucleic acid vectorBinding siteGC-content
The invention discloses a SgRNA for lowering immunogenicity, a low-immunogenicity dressing and a preparation method thereof. The sgRNA for lowering immunogenicity has a length from 18nt and 22nt; the3' end of the target sequence on the GGTA1 gene of the sgRNA contains GG, and the GC content is 40%-60%; The sgRNA does not have SNPs in the genome sequence of the target combination site; the sgRNA is compared with a pig GGTA1 gene to carry out the whole gene off-target effect analysis, and maximum 5 base mismatches are allowed in the off-target site. The low-immunogenicity dressing is pig skin,the pig skin is derived from a GGTA1 gene knockout pig. The sgRNA for lowering immunogenicity and the low-immunogenicity dressing have the advantages of low immunogenicity, even no immunogenicity, canbe used as a human skin substitute and reduce the risk of complications.
Owner:武汉博杰生物医学科技有限公司 +1
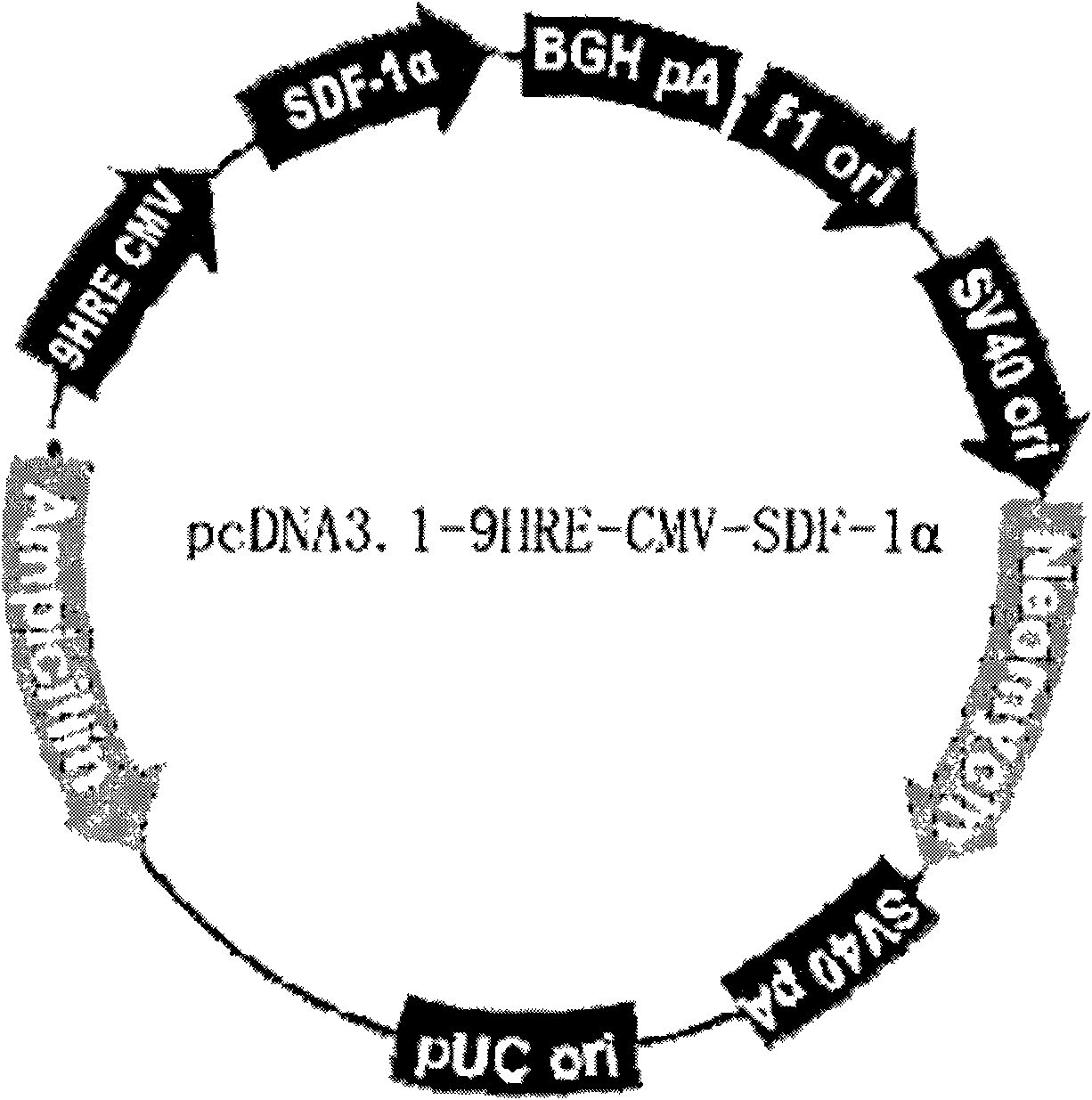
Skin substitute for automatically capturing endothelial progenitor cells and promoting vascularization and construction method thereof
InactiveCN101985052APromote vascularizationImprove securityFermentationVector-based foreign material introductionProgenitorCuticle
The invention relates to the technical field of tissue engineering and medical wound healing. The conventional skin substitute has low vascularization speed and low survival rate, and cannot be clinically popularized and applied after being grafted at present. The invention constructs a novel skin substitute capable of automatically capturing endothelial progenitor cells in peripheral blood and accelerating vascularization. A stroma cell derived factor 9HRE-CMV gene regulated by a hypoxic response element is used to transfect fibroblasts; epidermal cells and transgenic fibroblasts are implanted on both sides of a dermal scaffold respectively to form the sandwich skin substitute; and the skin substitute duly and moderately expresses and secretees SDF-1 alpha after being grafted, and endothelial progenitor cells (EPC) in the mediated peripheral blood are mediated to a skin substitute grafting place so as to promote the vascularization of the skin substitute and improve the survival rate after grafting. The skin substitute can obviously improve the chemotactic induction to the EPC and accelerates the vascularization of the skin substitute. The average survival rate of the skin substitute is 95 percent, while the average survival rate of the conventional skin substitute not containing the transgenic fibroblasts is 72 percent.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
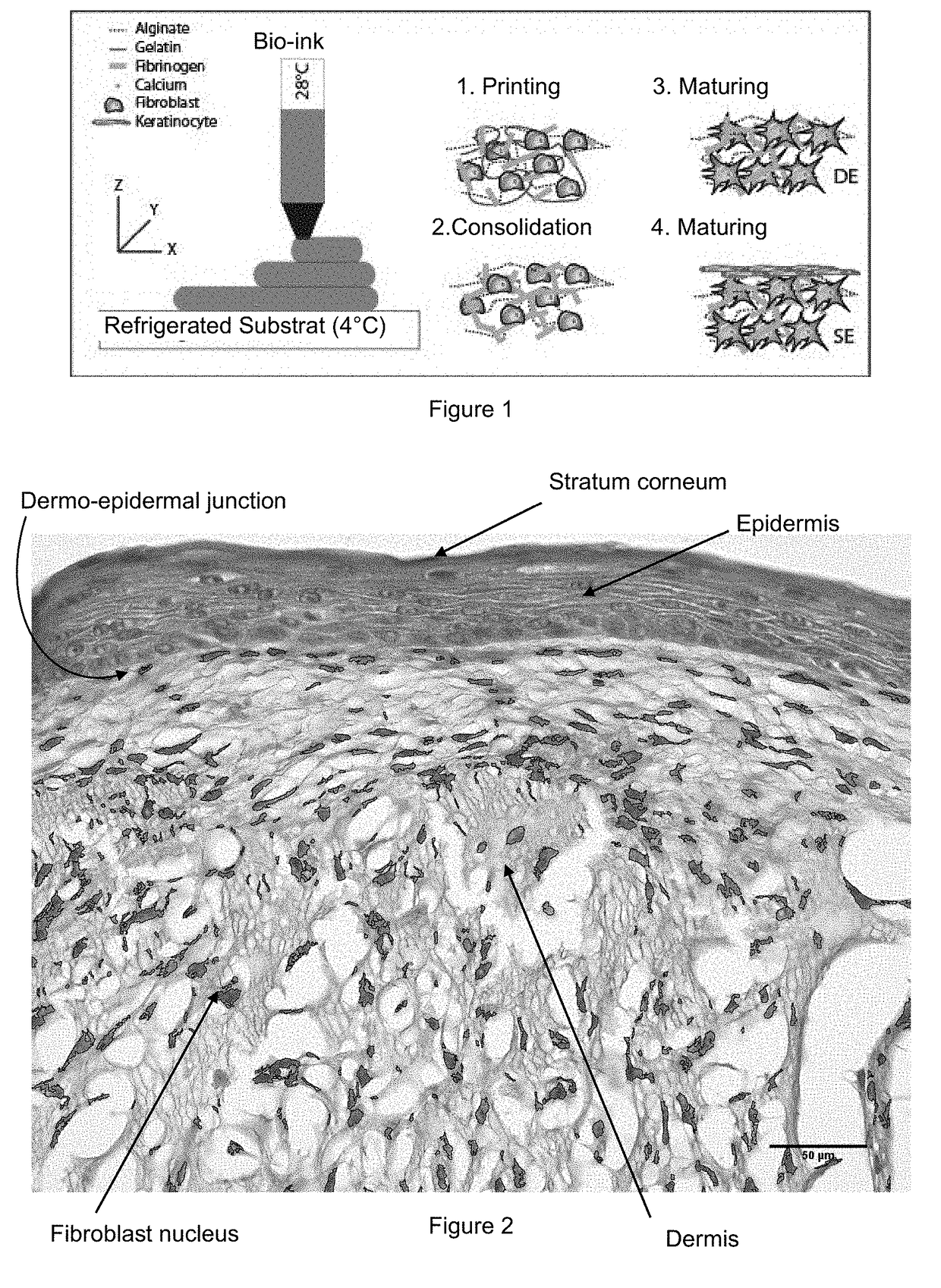
Method for Manufacturing Body Substitutes by Additive Deposition
The invention relates to a method for manufacturing a bio-ink by additive deposition, which comprises supplying: a first solution including between 5 and 40 wt. % gelatin; a second solution including between 15 and 35 .wt. % alginate; a third solution including between 1 and 15 wt. % fibrinogen, and optionally living cells in suspension; and creating a mixture including: around 35 to 65 vol. % of the first solution; around 15 to 35 vol. % of the second solution; and around 15 to 35 vol. % of the third solution, said proportions being selected so that they add up to 100%. Said bio-ink allows the additive deposition of objects that can be polymerised by means of a solution including calcium ions and thrombin. Said objects can be incubated and can be used as a substitute for body tissue, for example (with added fibroblasts) as skin substitute.
Owner:LAB SKIN CREATIONS +4
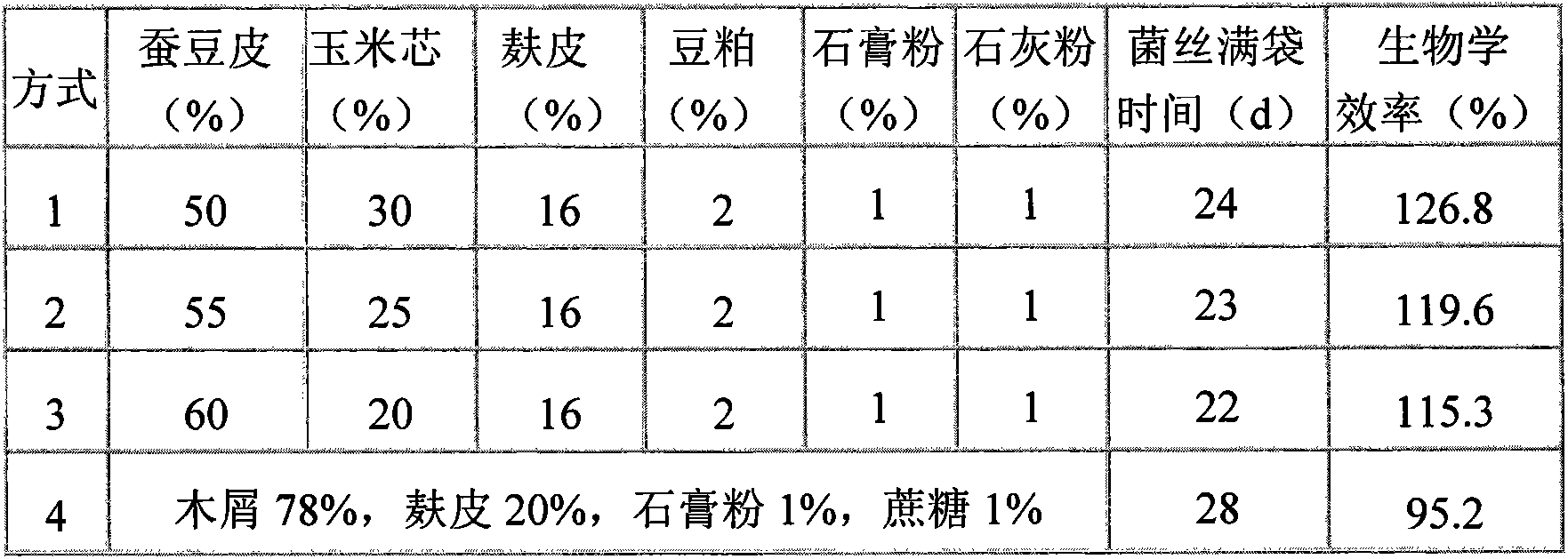
Compatibility of cultivation material for russula and manufacturing method of cultivation material
InactiveCN103396236AImprove breathabilityImprove water retentionFertilizer mixturesMushroomBroad beans
The invention relates to a cultivation material for russula, and the cultivation material is characterized in that: broad bean skin is used as a main raw material; corncob, wheat bran, soybean meal, gypsum powder and other auxiliary materials are supplemented, and the water content of the cultivation material is 63%-65%. The invention also includes a manufacturing method of the cultivation material. Compared with sawdust cultivation materials, the cultivation material has the advantages that: first, the cultivation material has a good air permeability and a strong water-retaining property, russula mycelium growth speed is rapid, and bag full-filling time is shortened; second, the cultivation material has a reasonable carbon nitrogen ratio and balanced nutrition, and can promote the growth and development of russula fruit body, and the yield is increased by 20%-30%; third, channels of cultivation raw materials are expanded, and the cost of production is greatly reduced. The social benefits of the cultivation material for the russula are that: through using a broad bean skin substitute material for cultivation of the russula, a part of ''mushroom-forest'' contradictions can be eased, waste materials can be recycled, and the cultivation material is conducive to energy saving and emission reduction.
Owner:邬金飞
Skin substitutes and methods for hair follicle neogenesis
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Mesenchymal stem cell tissue engineering scaffold and preparation method and application thereof
InactiveCN105816915ASpeed up wound regenerationImprove transplanting efficiencyProsthesisMesenchymal stem cellGelatin
The invention relates to the field of biological products and discloses a mesenchymal stem cell tissue engineering scaffold, a preparation method of the scaffold and application of the scaffold in skin substitutes. The preparation method of the scaffold comprises the steps of preparing a gelatin-chitosan porous scaffold and then inoculating the mesenchymal stem cells to the gelatin-chitosan porous scaffold for incubation and cultivation. The mesenchymal stem cell tissue engineering scaffold can obviously and effectively promote regeneration, reconstruction, repair and healing of skin wound surfaces difficult to heal, such as diabetes.
Owner:GENERAL HOSPITAL OF PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com