SgRNA for lowering immunogenicity, low-immunogenicity dressing and preparation method thereof
An immunogenicity and carrier technology, which is applied in the field of low immunogenicity biological dressings and its preparation, can solve the problems of high immunogenicity, reduction of immunogenicity, multiple dressing changes and scab removal, and achieve low immunogenicity, The effect of risk reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
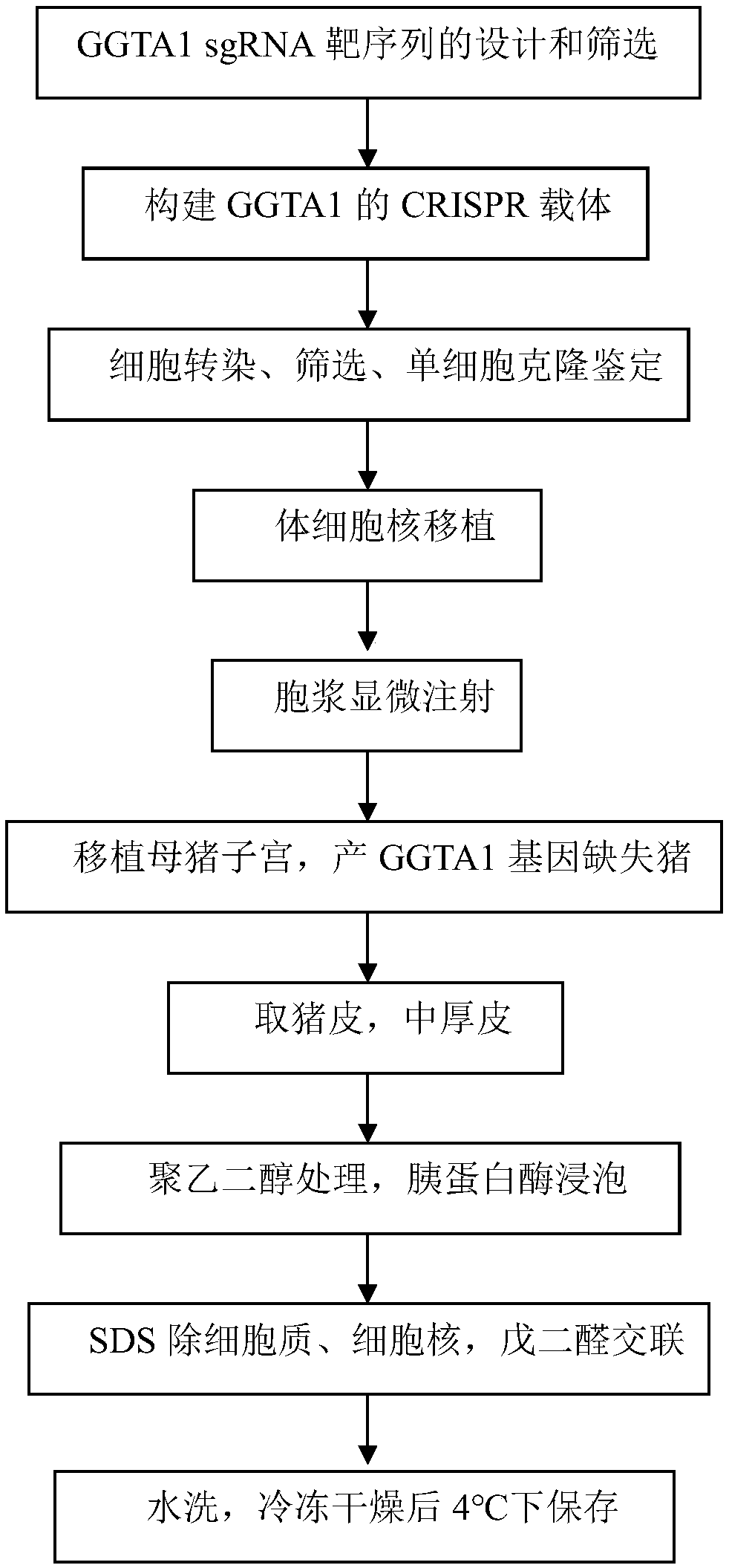
[0058] The preparation method of the dressing with low immunogenicity provided by the invention is characterized in that, comprising the following steps:
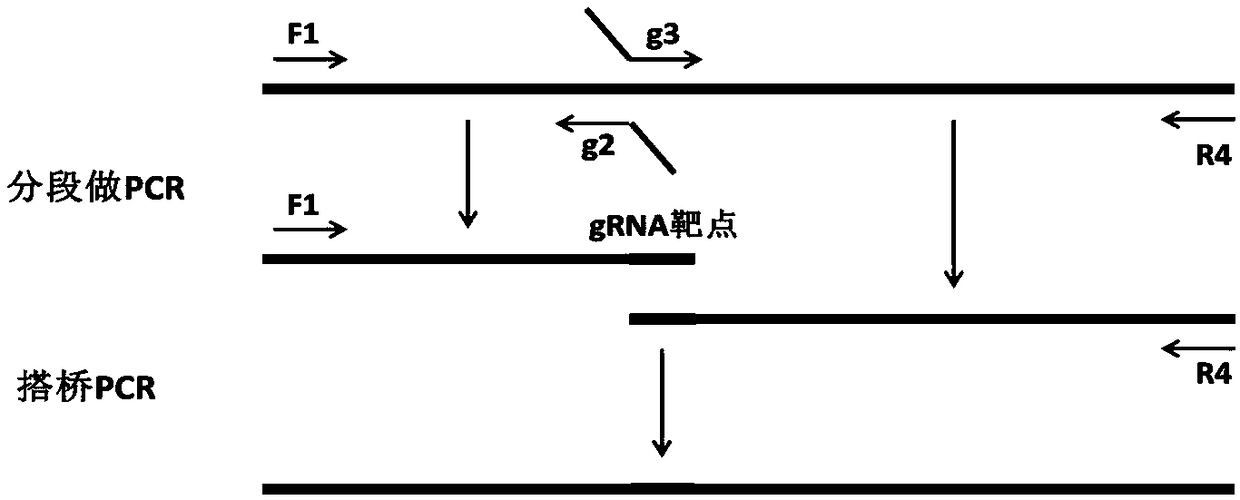
[0059] (1) Preparation of transfection vector: add RNA sequence TAGG to the 5' end of the sense strand template of the sgRNA target sequence provided by the present invention, add CAAA to the 5' end of the antisense strand template, complement the cohesive end of the vector plasmid, and synthesize Double strands formed by annealing single strands of oligonucleotide chains; The CRISPR / Cas backbone plasmid was digested with restriction endonuclease BbsⅠ, and the digested plasmid was purified; the linearized plasmid was ligated with sgRNA oligonucleotide double strands, transformed, sequenced, and extracted endotoxin-free plasmid for Cell transfection. Connect the linearized transfection vector CRISPR / Cas backbone plasmid and the in vitro transcription CRISPR / Cas backbone plasmid to the double strands respectively to obtain t...
Embodiment 1
[0071] Example 1. Design and screening of GGTA1 gene sgRNA and preparation of CRISPR / Cas9 system vector
[0072] 1. Design of sgRNA
[0073] For the GGTA1 gene, the principles of sgRNA design are as follows:
[0074] (1) The length is about 20nt;
[0075] (2) sgRNA containing GG at the 3' end, with a GC content of 40%-60%;
[0076] (3) There are no SNPs in the genome sequence of the sgRNA targeting binding site;
[0077] (4) Analysis of gene-wide off-target effects, a maximum of 5 base mismatches are allowed at off-target sites.
[0078] Based on the above design principles, the target sequence set shown in Table 1 was designed.
[0079] The sequence of the sgRNA designed in Table 1 (the gray mark is the PAM sequence)
[0080]
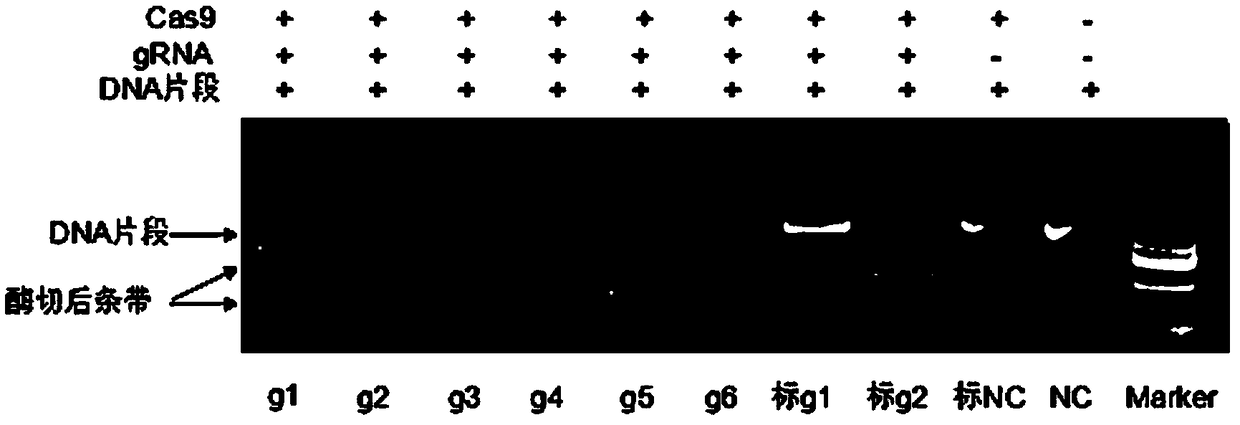
[0081] 2. Screening of sgRNA and preparation of in vitro transcription vector
[0082] According to the above principles, 6 suitable sgRNAs were selected, and the gRNA target efficiency was further detected by in vitro enzyme digestion activit...
Embodiment 2
[0124] Embodiment two, transfection adult fibroblast
[0125] 1. Cell transfection and screening
[0126] The U6-gRNA-3 plasmid was electroporated to transfect porcine adult fibroblasts. Follow the specific steps below:
[0127] ①Observe the growth status of the cells under a microscope, select cells that grow well and have a confluence of about 80%, digest with 0.25% trypsin for 2-3min, centrifuge at 1500g for 5min, and discard the supernatant;
[0128] ②Suspend the cell pellet in 500uL DPBS buffer, centrifuge at 1500g for 5min, and thoroughly wash away the residual trypsin in the cells;
[0129] ③ Add a certain volume of electroporation buffer to suspend the above cells, divide the electroporation solution into each group of centrifuge tubes according to the electroporation system size of 50uL, and add a certain mass of corresponding electroporation plasmid to it, and mix the cell suspension with a pipette gun;
[0130] ④ Turn on the ECM2001Elector Cell ManipuLator, and s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com