Turbidimetric immunoassay for assessing human cysteine proteinase inhibitor C
A technology of cysteine protease and immunoassay, which is applied to measuring devices, instruments, scientific instruments, etc., to achieve the effects of reducing measurement time, improving linearity, and reducing interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
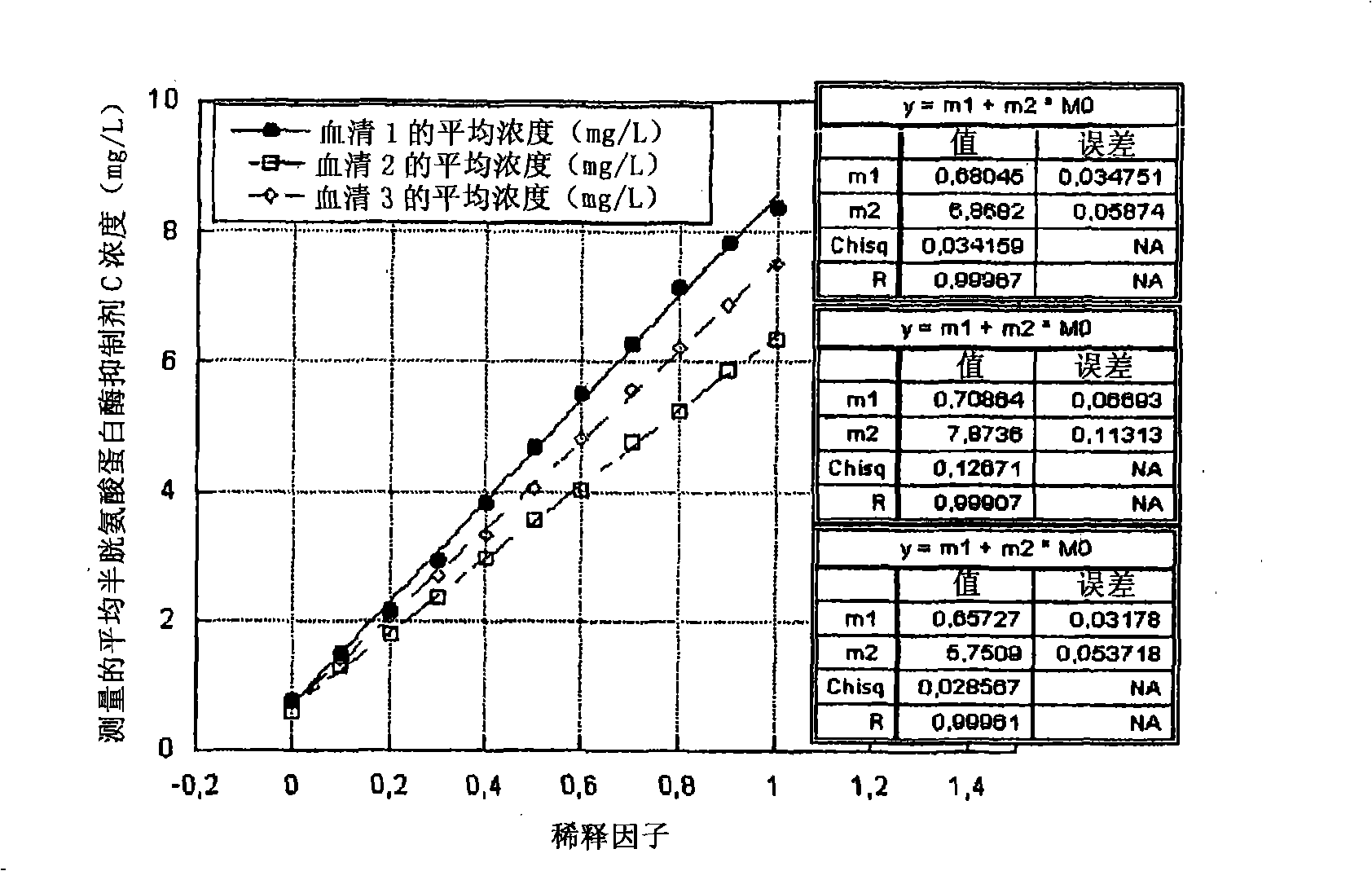
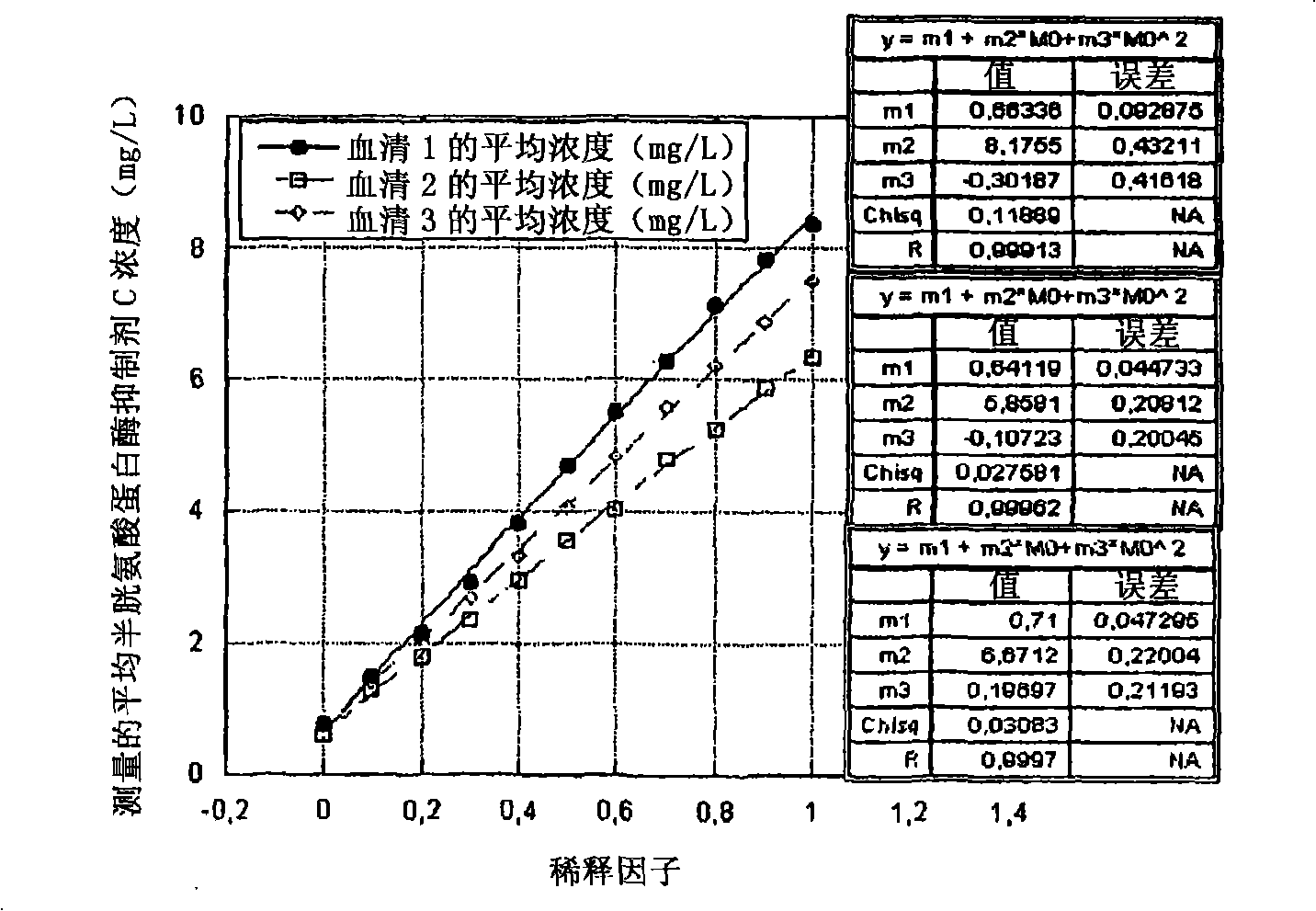
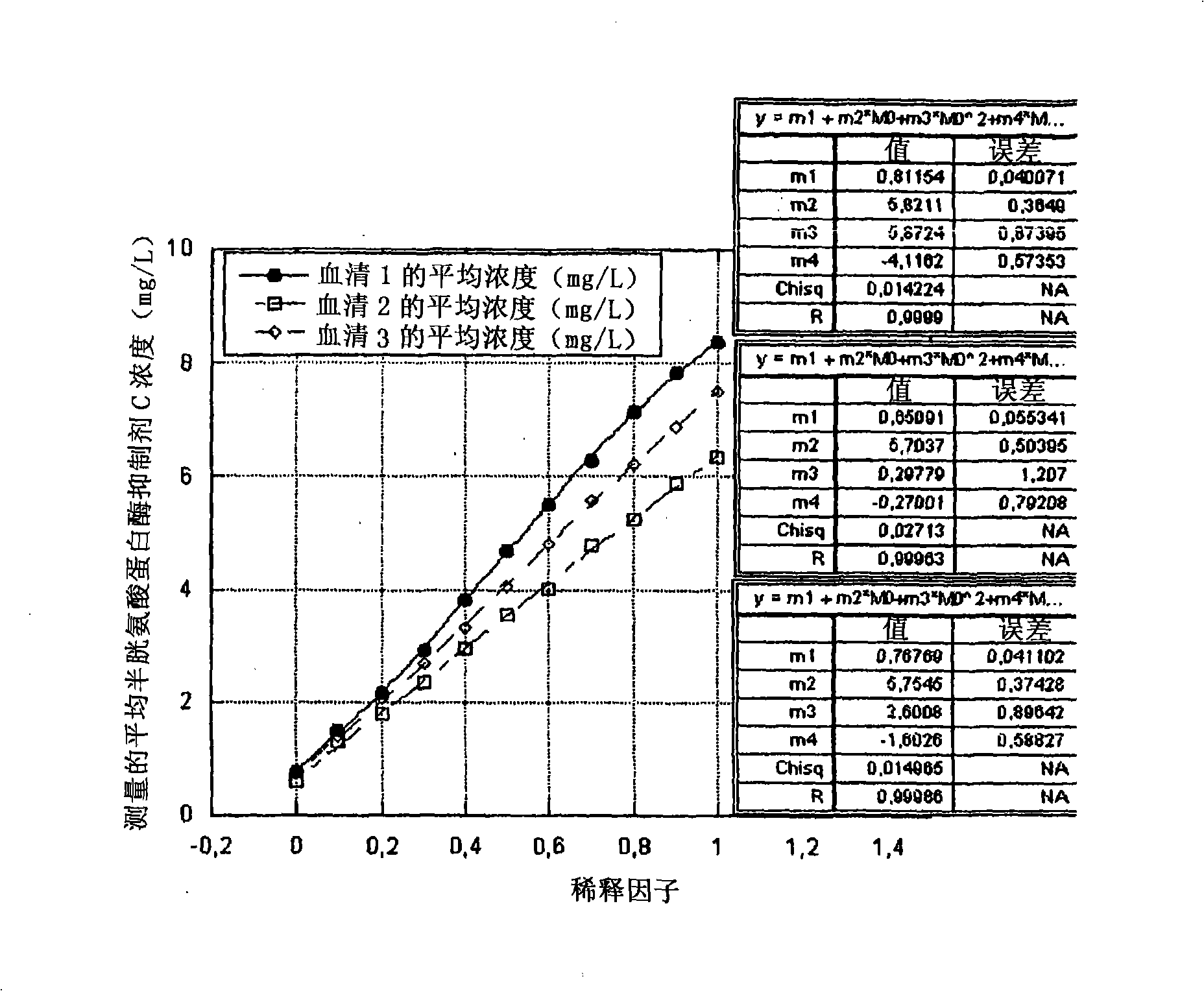
Image
Examples
Embodiment 1
[0263] Synthesis Example 1: Preparation of polyclonal poultry serum and its affinity purification
[0264] a) Preparation of poultry serum
[0265] Polyclonal IgY antibodies to cystatin C can be produced using the following immunization methods:
[0266] Use 2-4 hens per immunization experiment. Before immunization begins, a pair of eggs is collected. IgY purified from these eggs will serve as control IgY. 100 μg of highly purified human cystatin C (purified from the urine of patients with renal tubular proteinuria) in phosphate buffer was emulsified with Freund's complete adjuvant and injected into the breast muscle of hens middle. Injections were repeated every 4 weeks. 10 weeks after initiation of injections, eggs were collected. Egg yolk was isolated from the egg, and the IgY fraction from the egg yolk was isolated by ammonium chloride precipitation in a conventional manner following prior art methods of egg antibody isolation (see, e.g., Larsson A, Baaloew R-M, Lind...
Embodiment 2
[0270] Synthesis Example 2: Preparation of Monoclonal Anti-Human Cystatin C Antibody
[0271] Preparation of monoclonal anti-human cystatin C antibodies can be carried out as follows, by utilizing methods well known in the art, for example, from Harlow et al., 1988, "Antibodies: a Laboratory Manual" "Section 6, Cold Spring Harbor Press, New York, USA. Human PSA was isolated from human semen plasma as described by Sensabaugh et al., 1990, J. Urology 144, 1523.
[0272] Mice were immunized with 4 injections of 50 μg human Cystatin C in RAS (RIBI Adjuvant System) at regular intervals. Four months after the first injection, spleens from immunized mice were isolated using the polyethylene glycol method described by G. Galfre et al., 1981, Methods in Enzymology, 73, 3-46. Lymphocytes were fused with the myeloma cell line SP2 / 0-Ag14.
[0273] Hybridomas secreting antibodies against cystatin C were identified by the following screening ELISA: microtiter plates were coated with rabb...
Embodiment 3
[0277] Synthesis Example 3: Preparation of Anti-cystatin C Immune Particles
[0278] a) Coupling method - standard method:
[0279] (1) Buffers and reagents
[0280]
[0281] In order to have more signal / mg antibody from the polymer particles, the pH of the coupling buffer is adapted in a special way to the pi of the antibody to be coupled. Additionally, the antibodies were diluted with albumin / transferrin prior to conjugation (see below). For conjugation, when using monoclonal antibodies, choose a pH that is half a unit above the pi of the antibody. Polyclonal antibodies have a very variable pi, and therefore a pH 8.8 polyclonal antibody was used. The selected pH was obtained by mixing the above mentioned PBS and borate buffer.
[0282] The following standard methods outline two simple one-step methods for attachment by physical adsorption of antibodies to chloromethyl groups. The method was used for a 1 μm chloromethyl latex at 1% solids. This reaction can be easi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com