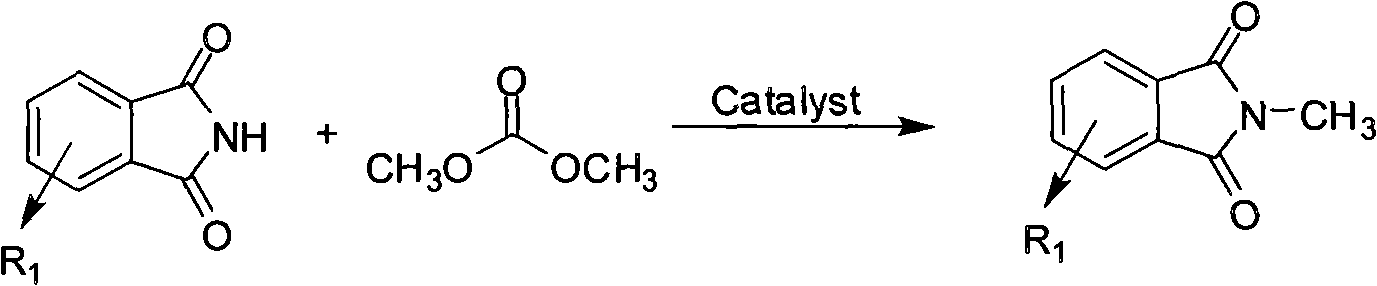
Method for preparing N-methyl phthalimide compound
A technology of methyl phthalimide and phthalimide, which is applied in the field of preparation of N-methyl phthalimide compounds, can solve problems such as pressurization equipment and the like, and achieve reaction The effect of short time, low production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] In the reaction flask, add 73.5 grams (0.5 moles) of phthalimide, 2.85 grams (25 mmoles) of 1,4-diazabicyclo[2.2.2] octane (DABCO), dimethyl carbonate 500 milliliters of esters were stirred and refluxed for reaction, followed by TLC (thin layer chromatography) until the raw material point disappeared, and it took about 7 hours. After the reaction was completed, dimethyl carbonate was distilled off under reduced pressure, 400 ml of water was added, stirred for 10 minutes, and a white solid was obtained by filtration, which was recrystallized with ethanol to obtain 72.8 g of white crystals with a melting point of 132-134°C. The amount of feeding amount and the amount of N-methylphthalimide obtained, the calculated yield is 91%.
[0030] The properties of N-methylphthalimide are as follows:
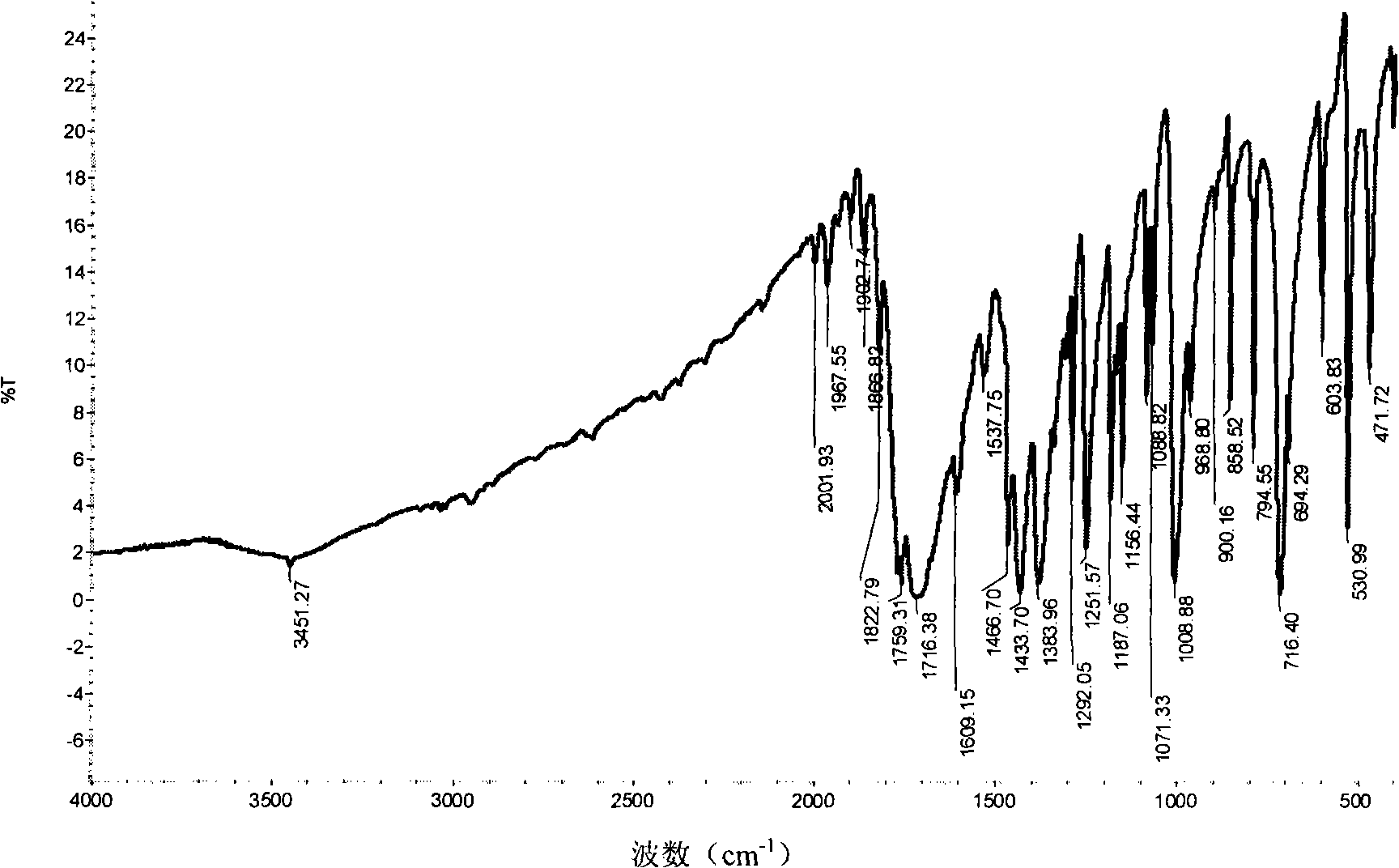
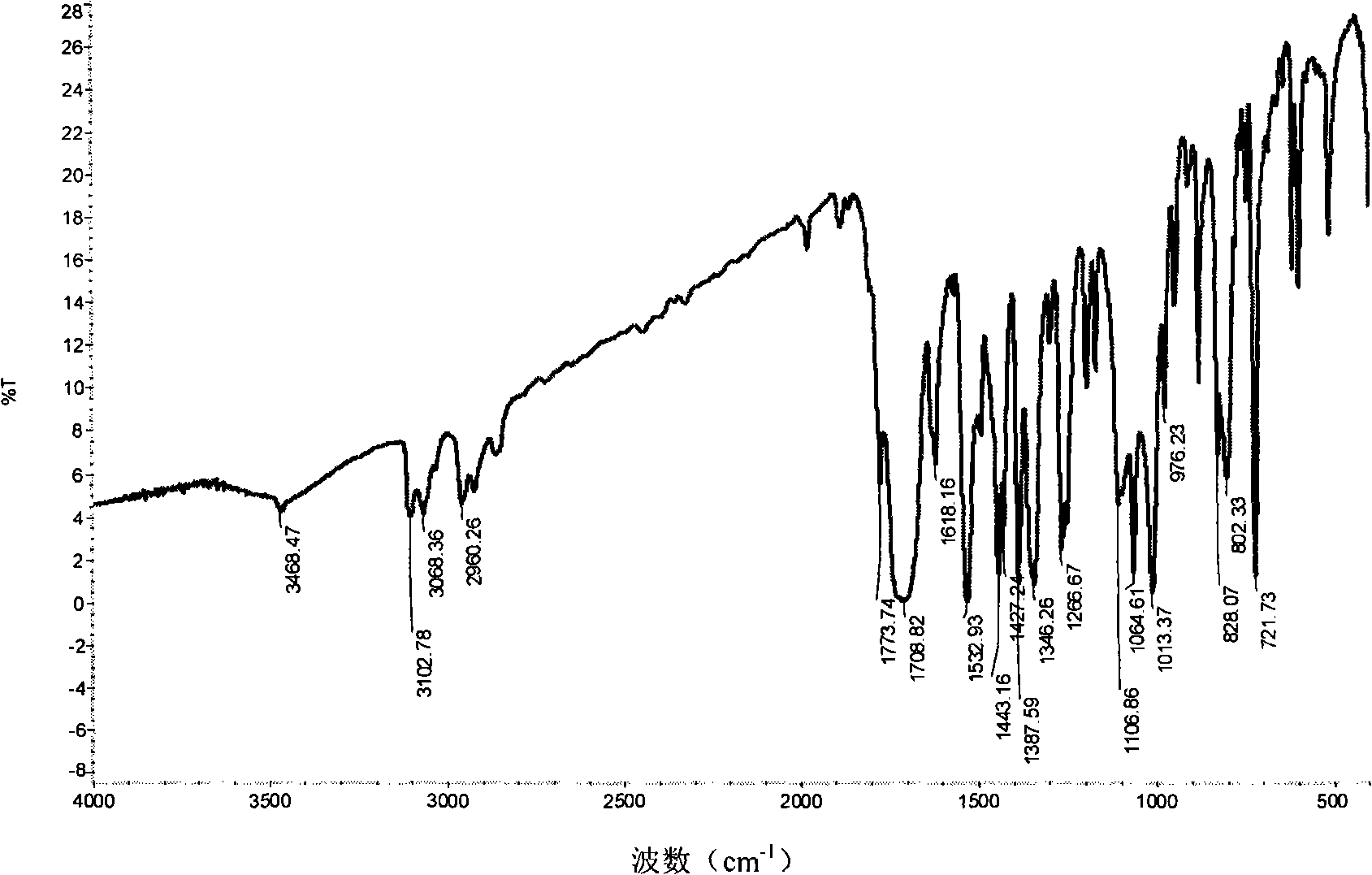
[0031] IR(KBr)υ: 3105, 2978, 1716, 1609, 1466, 1383, 1292, 1251, 1187, 1156, 1088, 1008cm -1 , 3105cm in the infrared IR spectrum -1 Nearby are the C-H stretching vibra...
Embodiment 2
[0034]
[0035] In the reaction flask, add 73.5 grams (0.5 moles) of phthalimide, 2.85 grams (25 mmoles) of 1,4-diazabicyclo[2.2.2] octane (DABCO), dimethyl carbonate 50 ml of ester and 250 ml of dimethylformamide (DMF) were stirred and refluxed at 110° C., followed by TLC (thin-layer chromatography) until the starting point disappeared, and it took about 5 hours. After the reaction was completed, the dimethyl carbonate was distilled off under reduced pressure, 300 ml of water was added, and extracted with ethyl acetate (200 ml×3). Ethyl acetate was distilled off, and the residue was recrystallized with ethanol to obtain 71.3 g of white crystals with a melting point of 132-134°C. amount, the calculated yield was 89%.
Embodiment 3
[0037]
[0038] Except that 1,4-diazabicyclo[2.2.2]octane (DABCO) in Example 1 was replaced with tetramethylethylenediamine, all the other raw materials, formulations, processes and operating procedures were the same as in Example 1. Obtain 68.4 grams of white N-methylphthalimide crystals, according to the amount of phthalimide charging and the amount of N-methylphthalimide obtained, the calculated yield is 85 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com