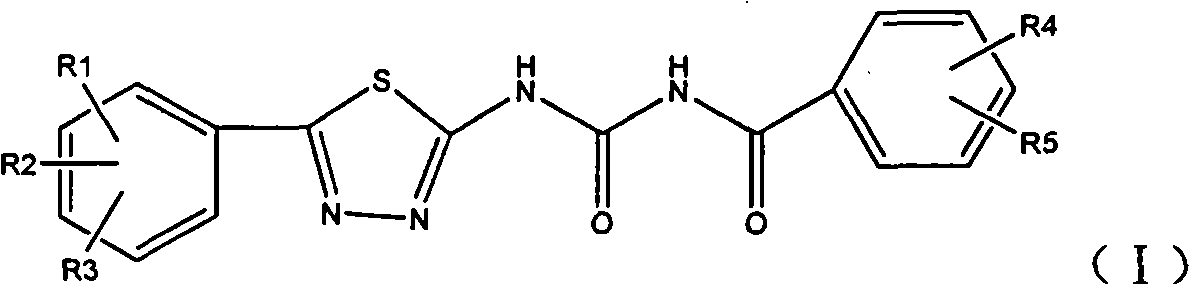
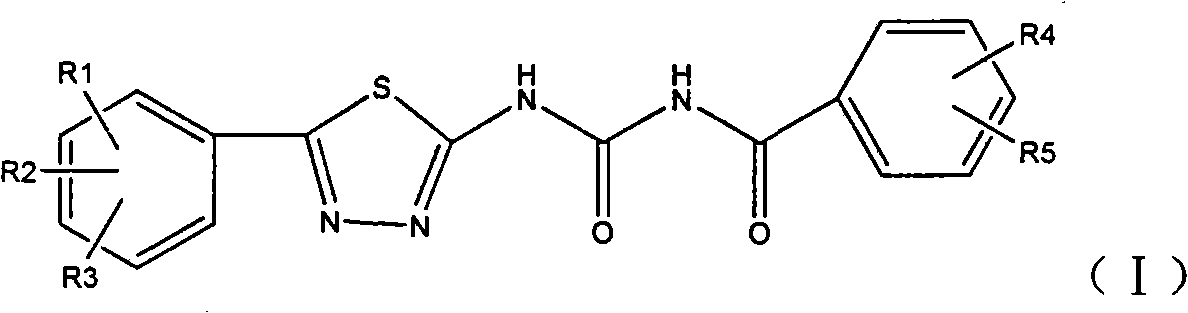
1,3,4-thiadiazole aroyl urea compound, preparation method and application thereof
A compound and thiadiazole technology, applied in 1 field, can solve the problems of human health hazards, ecological environment pollution, backward production supervision and management, etc., and achieve the effects of good sterilization effect, broad market prospect and simple process method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
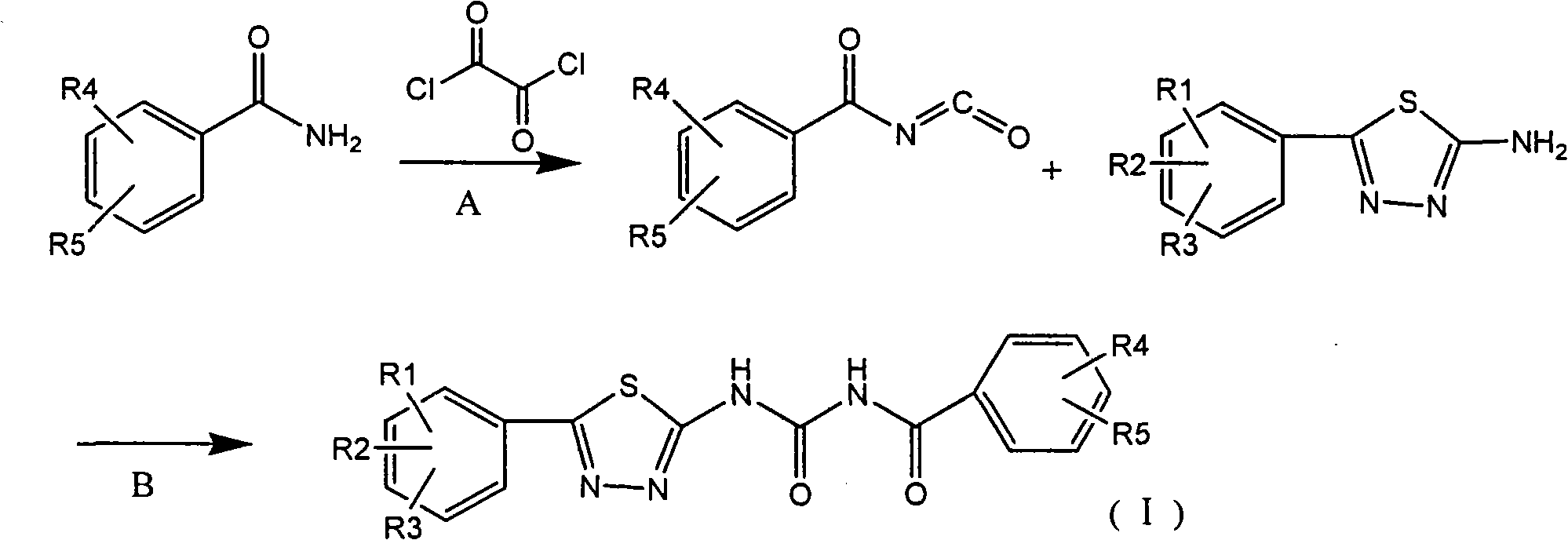
Examples
Embodiment 1
[0056] Embodiment 1 2, the preparation of 6-difluorobenzoyl isocyanate
[0057] At room temperature, place 0.24mol of oxalyl chloride, 120mL of 1,2-dichloroethane and 0.1mol of 2,6-difluorobenzamide in a 250mL four-necked flask equipped with a reflux condenser, and install a drying tube and tail gas Absorption device, it takes about 5 hours to react under electromagnetic stirring until no gas is produced. The 1,2-dichloroethane and oxalyl chloride were distilled off first under normal pressure and then under reduced pressure, and the residue was a colorless oily liquid sealed in a desiccator for later use.
Embodiment 2
[0058] Example 2 Preparation of 2,6-difluoro-N-[5-(4-nitro)-1,3,4-thiadiazole-2-amino]benzamide
[0059] In a 250mL four-necked flask equipped with mechanical stirring and dropping funnel, add 0.01mol 2-amino-5-(4-nitrophenyl)-1,3,4-thiadiazole and 60ml toluene, 0.014mol of 2,6-difluorobenzoyl isocyanate prepared in Synthesis Example 1 was added dropwise, stirred and reacted, and detected by TLC until the reaction was complete, which took about 12 hours. After filtration, the filtrate was evaporated to remove part of the solvent and then cooled to obtain the crude product, which was recrystallized from DMF to obtain a pinkish-yellow powder (1.1 g, yield 76%), melting point: 258-259°C.
[0060] 1 H NMR: 7.26-8.60 (7H, m), 11.81 (1H, s), 12.13 (1H, s).
Embodiment 3
[0061] Example 3 Preparation of 2,6-difluoro-N-[5-(4-methoxy)-1,3,4-thiadiazole-2-amino]benzamide
[0062] In a 250 mL four-necked flask equipped with mechanical stirring and dropping funnel, add 0.01 mol of 2-amino-5-(4-methoxyphenyl)-1,3,4-thiadiazole and 80 mL of toluene, and 0.014mol of 2,6-difluorobenzoyl isocyanate prepared in Synthesis Example 1 was added dropwise, stirred and reacted, and detected by TLC until the reaction was complete, which took about 8 hours. After filtering, the filtrate was evaporated to remove part of the solvent and then cooled to obtain the crude product, which was recrystallized from ethanol to obtain white crystals (1.4 g, yield 83%), melting point: 203-205°C.
[0063] 1 H NMR: 3.84 (3H, s), 7.07-7.90 (7H, m), 11.73 (2H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com