Medicaments composite for treating gland hyperplasia and breast cancer and preparation method thereof
A technology for mammary gland hyperplasia and breast cancer, which is applied in the treatment of mammary gland hyperplasia, traditional Chinese medicine composition and its preparation for the treatment of breast disease, breast cancer granules and its preparation, and can solve the problem of limiting the curative effect of drug prescriptions and unreasonable preparation processes, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
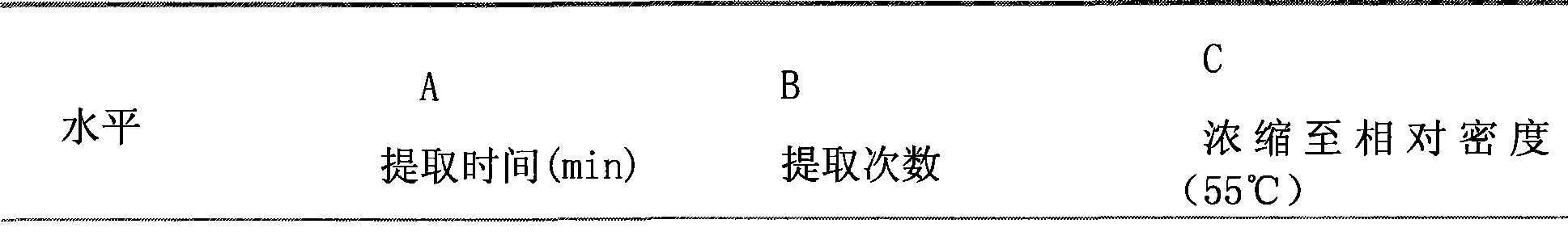
[0024] Experimental Example 1. Optimal experiment of the present invention's traditional Chinese medicine composition and substance extraction process
[0025] 1. Test material
[0026] Agilent1100 High Performance Liquid Chromatograph, including G1311A Quaternary Gradient Pump, G1313A Autosampler, G1315A Diode Array Detector (Hewlett Packard, USA); Lambda 25 UV / VIS spectrometer (PerkinElmer, USA); AG285 Analytical Balance (Meter, Germany) Le Company); KS-600D ultrasonic cleaning machine (Ningbo Kesheng Instrument Factory); 98-1-B electronic thermostat electric heating mantle (Tianjin Test Instrument Co., Ltd.). 5-Hydroxymethylfurfural reference substance (batch number: 111626-200402) was purchased from China Institute for the Control of Pharmaceutical and Biological Products, acetonitrile was chromatographically pure, and the rest of the reagents were of analytical grade.
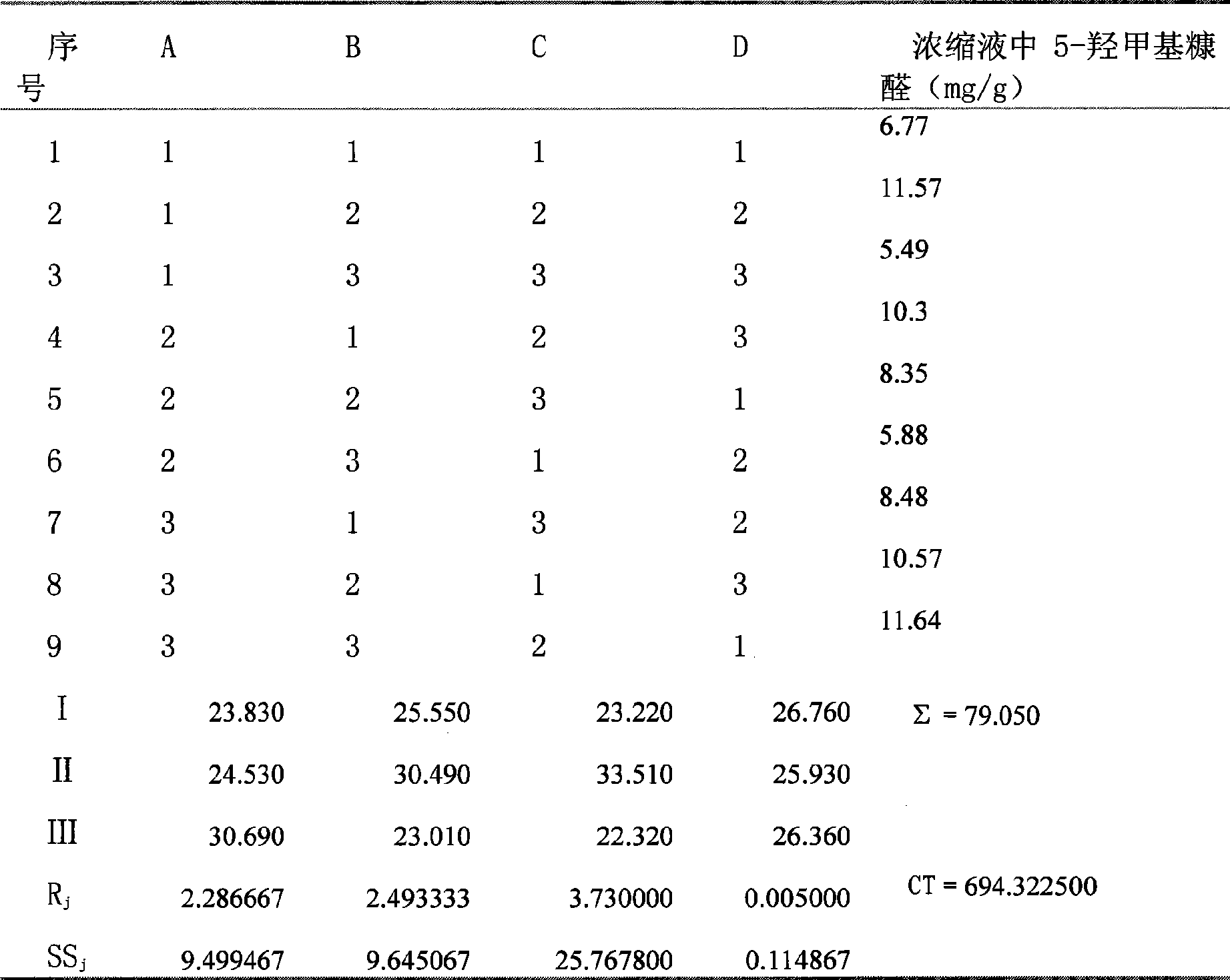
[0027] 2. Methods and Results
[0028] 2.1 Determination of 5-Hydroxymethylfurfural content by HPLC ...
experiment example 2
[0050] Experimental example 2. Screening of auxiliary materials in the preparation of capsules and granules of the present invention
[0051] The extract of the present invention contains more hygroscopic substances such as mucus and lipopolysaccharide, and the obtained solid preparation of the product is easy to absorb moisture during storage, which makes the product quality unstable; The appropriate excipients are selected according to the hygroscopicity, formability, meltability, bulk density and angle of repose of the pellets made by mixing the excipients with the extract powder.
[0052]Experimental method: Take 43.8g of Lithospermum, 175g of Shudi, 45.8g of Fuzi (made), 58.3g of Corydalis Corydalis, 58.3g of cinnamon in prescription quantities, and extract and concentrate the medicinal materials according to the best extraction process. 175g of melon, 87.5g of turtle shell, and 35g of whole scorpion were pulverized into fine powder, the extract of antler gum was mixed wi...
experiment example 3
[0098] Experimental Example 3. Preparation of the tablet of the present invention
[0099] Get the above-mentioned 1 / 2 prescription quantity of Lithospermum, and pulverize the melon, turtle shell, and whole scorpion into fine powder to obtain medicine fine powder, which is for subsequent use; the remaining medicinal flavors are boiled with water, combined with decoction, concentrated into extract, mixed with above-mentioned fine powder , and then mixed with sodium carboxymethyl starch with a fine powder content of 0.5-5% to obtain a drug mixture; after the antler gum is melted, it is added to the above mixture to make granules, dried, granulated, compressed into tablets, and coated to obtain .
[0100] The disintegration of the tablet made of the present invention is not good, so we screened the added disintegrant through experiments, and the test results are as follows in Table 12: Table 12 Experimental results of tablets made from different disintegrants
[0101] d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com