Method for preparing magnesian by calcining ammonium salt and magnesium-containing ore
A technology of magnesium ore and magnesium oxide, applied in lime production, etc., can solve problems such as high energy consumption, and achieve the effects of reducing energy consumption, reducing raw material consumption, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
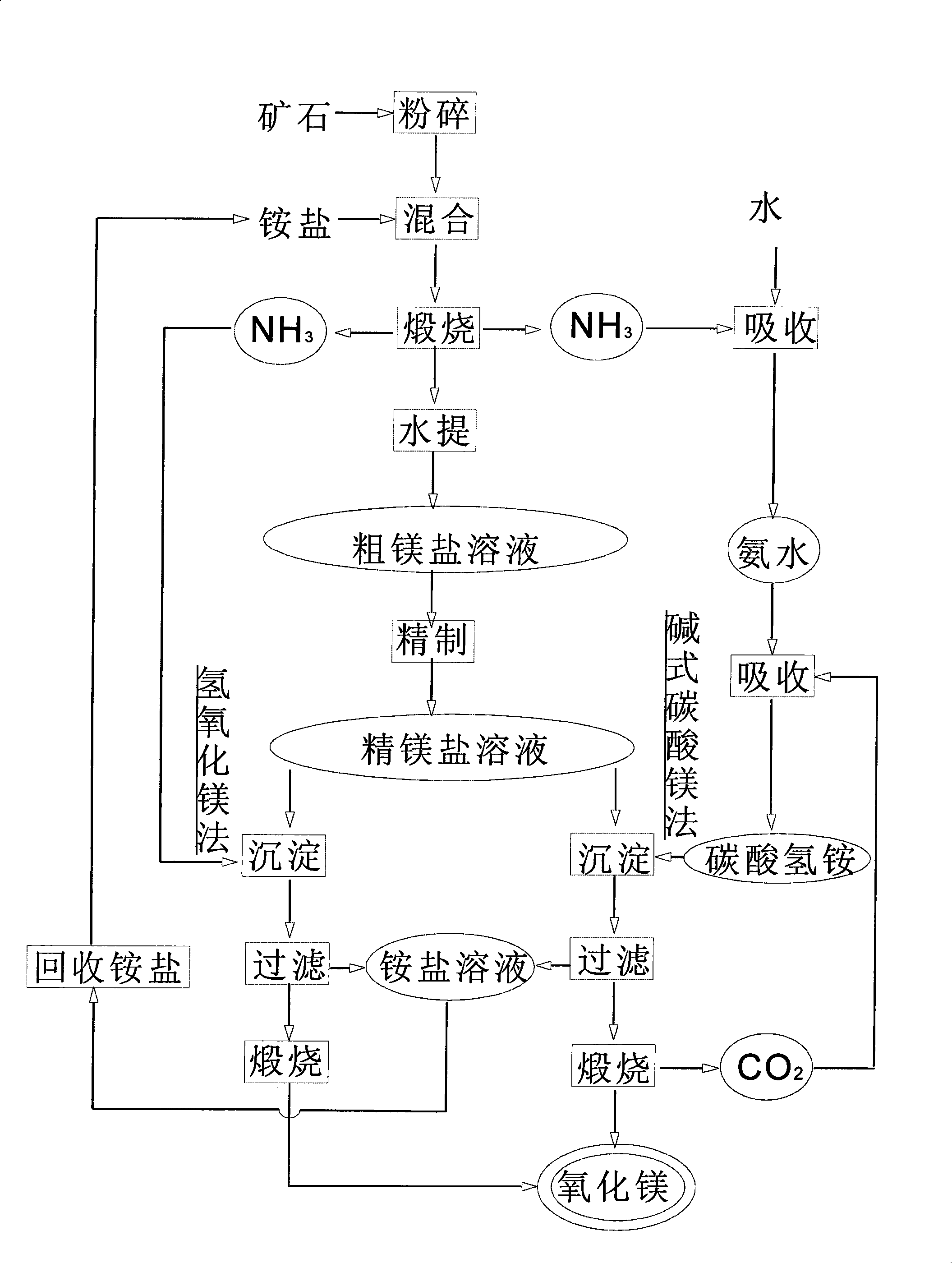
[0018] (1) 1 part of 100 mesh serpentine powder is mixed with 2.0 parts of 60 mesh ammonium chloride and put into the reactor with ammonia purifier and ammonia absorber, and the reactor is placed in the heating furnace and gradually heated to 300-350°C, when the ammonia produced by calcination is released, dissolve the leached and calcined product with 10 parts of water, filter to obtain a crude magnesium chloride solution, and recover the released ammonia;
[0019] (2) react with the ammonia that reclaims after adopting conventional method to refine thick magnesium chloride solution and obtain magnesium hydroxide precipitation;
[0020] (3) Washing and drying the precipitated magnesium hydroxide obtained by filtration is placed in a calciner, and calcined at 900-950° C. for 3 hours to obtain 0.35 parts of magnesium oxide;
[0021] (4) The ammonium chloride solution from which the magnesium hydroxide precipitate was separated was evaporated, concentrated and crystallized, and ...
Embodiment 2
[0022] Embodiment 2: Mix 1 part of 100 mesh dolomite powder with 1.6 parts of 100 mesh ammonium sulfate, other steps and process conditions are the same as in Example 1, and the ammonium salt recovered is ammonium sulfate.
Embodiment 3
[0024] (1) Mix 1 part of 100-mesh magnesite powder with 2.5 parts of 60-mesh ammonium chloride and put it into a reactor with ammonia gas purifier and ammonia absorber, and place the reactor in a heating furnace to gradually heat to 200°C, and finally raise the temperature to 350°C, when the ammonia produced by calcination is released, dissolve the leached and calcined product with 10 parts of water, filter to obtain a crude magnesium chloride solution, and dissolve the released ammonia with water to obtain ammonia water;
[0025] (2) first the carbon dioxide that calcined basic magnesium carbonate precipitation produces is inserted in ammoniacal liquor and obtains ammonium bicarbonate solution, then ammonium bicarbonate solution is added in the refined magnesium chloride solution and reacted to obtain basic magnesium carbonate precipitation;
[0026] (3) washing and drying the basic magnesium carbonate precipitate obtained by filtration, placing it in a calciner, and calcining...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com