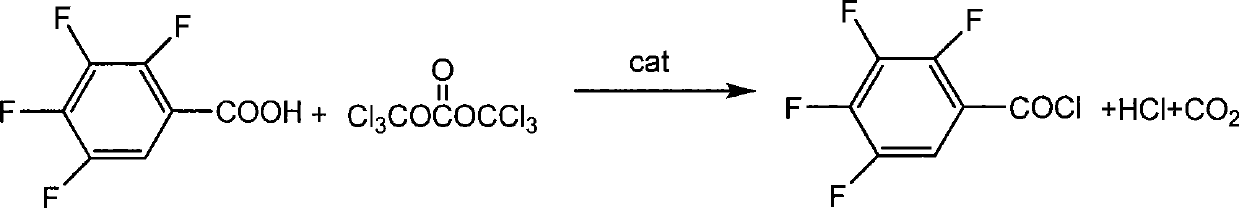
Chemical synthesis method of 2,3,4,5-phenyl tetrafluoride formyl chloride
A tetrafluorobenzoyl chloride, chemical synthesis technology, applied in 2 fields, can solve problems affecting product appearance and quality, high production cost, difficult handling, etc., achieve great implementation value and social and economic benefits, safe and reliable production, and process routes advanced effects
Active Publication Date: 2009-02-25
ZHEJIANG UNIV OF TECH +1
View PDF1 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The tail gas of the above-mentioned process contains a large amount of asphyxiating sulfur dioxide (one of the six indicators strictly controlled by the national environmental protection on the atmosphere), which is difficult to deal with. At present, the mixture of sodium sulfite and sodium chloride is mainly prepared by alkali absorption, but there are big problems in market sales. Moreover, because it is an aqueous solution after being absorbed by alkali, it must be concentrated to obtain a mixture of sodium sulfite and sodium chloride. The production process consumes a lot of energy and the production cost is relatively high.
At the same time, due to the concentrated release of sulfur dioxide during the reaction process, it is difficult to completely absorb it.
Enterprises in our country will inevitably face the fate of stopping production when adopting the thionyl chloride method. In addition, the transportation and use of thionyl chloride are strictly controlled, and the requirements for the sealing of the reaction equipment are high, and the investment is also large. At the same time, the products produced by the original process contain traces of sulfur dioxide gas. , the color of the product is easy to turn yellow during storage, which affects the appearance and quality of the product
At the same time, the original process also has problems such as low product yield and purity to be solved
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a method for chemical synthesis of 2, 3, 4, 5-tetrafluorobenzoyl chloride. The method comprises the following steps: 2, 3, 4, 5-tetrafluorobenzoic acid reacts with bis(trichloromethyl) carbonate in an organic solvent under the action of an organic amine catalyst at the temperature of 20-100 DEG C for 1-10h, and the 2, 3, 4, 5-tetrafluorobenzoyl chloride is obtained after post treatment; and the organic amine catalyst is tertiary amine containing N-formoxyl group or solid-phase supported tertiary amine containing the N-formoxyl group. The method has the advantages of mild reaction conditions, high reaction yield and less three wastes, and has greater implementation values, social benefits and economic benefits.
Description
(1) Technical field The invention relates to a chemical synthesis method of 2,3,4,5-tetrafluorobenzoyl chloride. (2) Background technology 2,3,4,5-Tetrafluorobenzoyl chloride is an important intermediate used in the synthesis of chemical raw materials, such as the production of ofloxacin or levofloxacin with it. Before the present invention provides, the chemical synthesis method of 2,3,4,5-tetrafluorobenzoyl chloride in the prior art mostly is chlorinated with 2,3,4,5-tetrafluorobenzoic acid and thionyl chloride prepared. As proposed in US4777253, 14.5g of 2,3,4,5-tetrafluorobenzoic acid and 152ml of thionyl chloride were refluxed for 4 hours under the catalysis of a small amount of DMF. The tail gas of the above-mentioned process contains a large amount of asphyxiating sulfur dioxide (one of the six indicators strictly controlled by the national environmental protection on the atmosphere), which is difficult to deal with. At present, the mixture of sodium sulfite and so...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C63/10C07C51/60
Inventor 苏为科陈志卫杨郭明姜灵陈寅镐王超
Owner ZHEJIANG UNIV OF TECH
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com