Electrocatalysts having gold monolayers on platinum nanoparticle cores, and uses thereof
一种电催化剂、单层的技术,应用在纳米技术、纳米技术、电化学发生器等方向,达到减少装载的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0204] Preparation of gold-coated platinum nanoparticles
[0205] Gold-coated platinum nanoparticles (also denoted Au / Pt) were prepared by depositing a monoatomic layer of gold on the platinum nanoparticles. Gold was deposited using a redox displacement method as described by Zhang et al. (supra).
[0206] In this method, platinum nanoparticles are placed on a suitable electrode and immersed in ~50mM CuSO 4 / 0.10MH 2 SO 4 Applying a suitable reduction potential to this electrode in aqueous solution deposits a copper monolayer (coppor adlayer) onto platinum nanoparticles with a diameter of about 3-10 nm. The electrode with copper-coated Pt nanoparticles was rinsed with clean water to move copper(2+) ions into solution. To replace the copper monolayer with a gold monolayer, the electrode with Pt nanoparticles is subsequently immersed in a suitable gold salt such as HAuCl 4 ) in ~1.0mM aqueous solution. After 1-2 minutes of immersion, the copper is completely replaced by ...
Embodiment 2
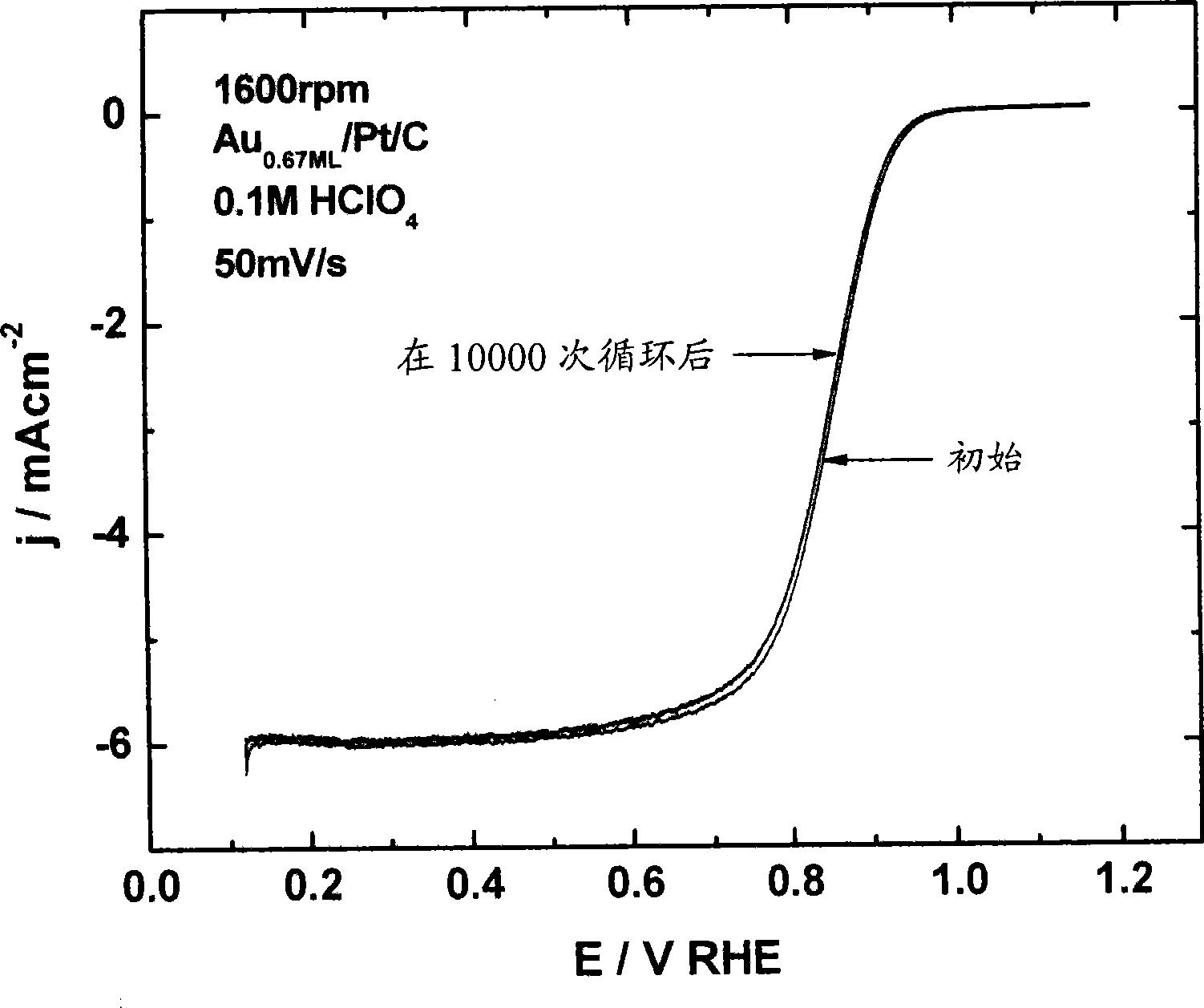
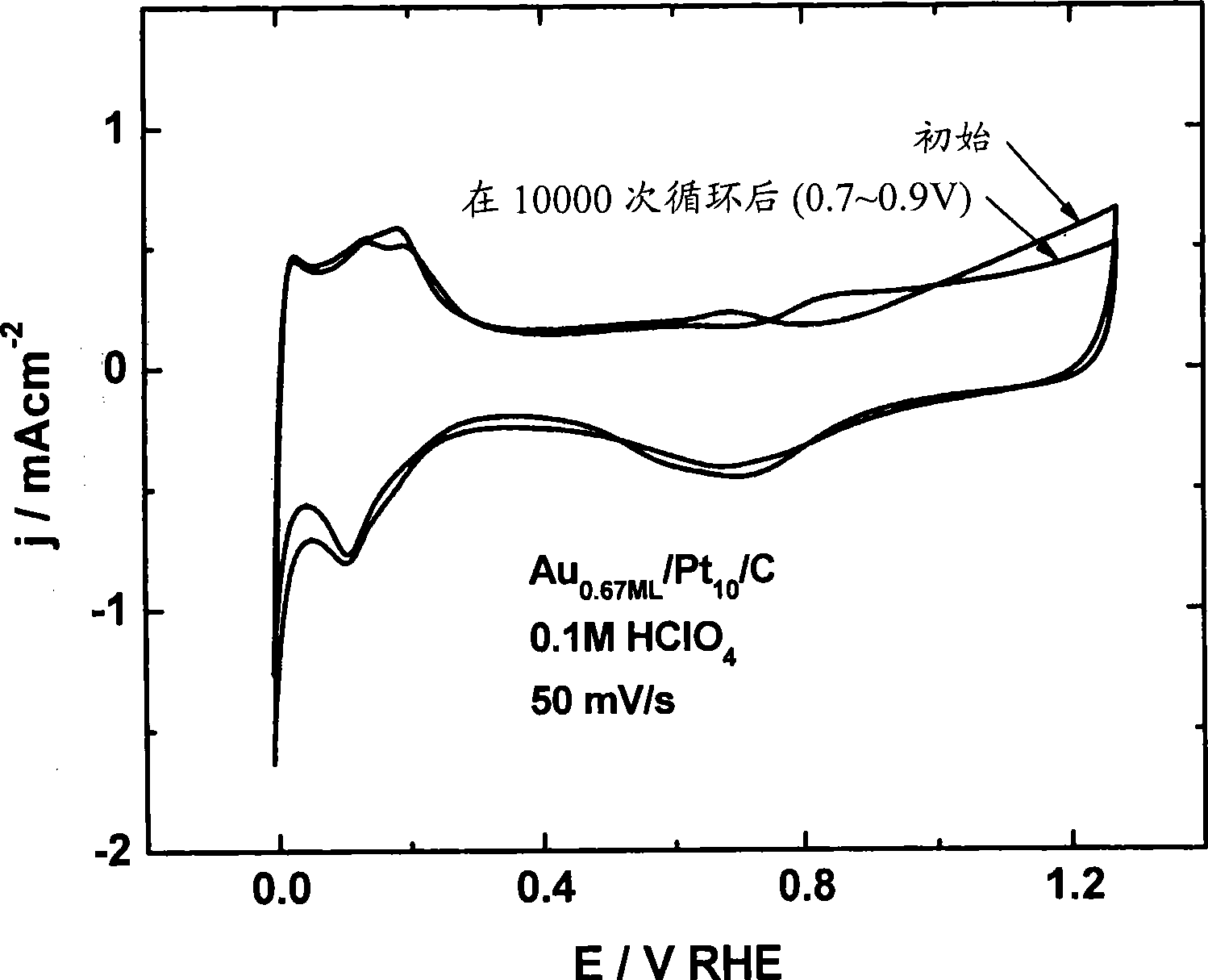
[0210] Electrocatalytic Activity Measurement of Platinum Nanoparticles Coated with Gold Monolayer
[0211] Dissolution of platinum from platinum electrocatalyst nanoparticles on an oxygen-reducing cathode can be prevented by placing a submonolayer-to-monolayer of gold on platinum nanoparticles. Because gold is oxidized at a much more positive potential than platinum (Pt is 0.75 V compared to Au's 1.3 V), it can be confirmed that gold-coated platinum electrocatalysts have positively (benignly, positively) transferred platinum oxidation. This forward-transferred platinum oxidation leads to the enhanced stability observed for gold-coated platinum electrocatalysts.
[0212] In addition to the remarkable stability, surprisingly, the oxygen reduction of submonolayer-to-monolayer gold-covered platinum occurs with almost exactly the same kinetics as platinum. Therefore, the electrocatalytic activity of platinum in these gold-coated electrocatalysts remains intact and is not damage...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com