Diabecron sustained-release tablet and preparation method thereof
A technology of metformin hydrochloride and sustained-release tablets, which is applied to the oral drug metformin hydrochloride for treating diabetes and its preparation, and the field of sustained-release tablets and its preparation, can solve the problem that the timed and quantitative release of drugs and release can not be achieved well. Large difference, instability and other problems, to achieve the effect of reliable treatment and self-prevention of sudden onset, small difference in release, and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
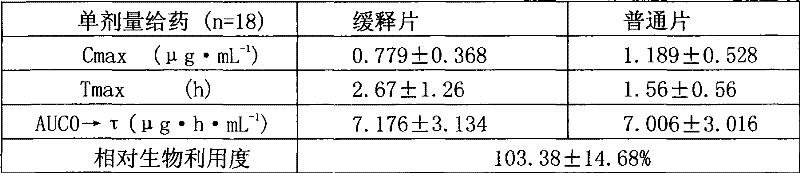
[0040] The metformin hydrochloride sustained-release tablet includes a main drug, a sustained-release material and other auxiliary materials, and is characterized by being formulated in the following weight percentages: 68% of the main drug, 19% of sustained-release materials, and 13% of other auxiliary materials.
[0041] The sustained-release tablet is prepared from the following raw materials in weight ratio:
[0042] 1000 tablets prescription
[0043] Metformin hydrochloride 68%
[0044] Hypromellose K100 16.3%
[0045] Glyceryl behenate 2.7%
[0046] Lactose 6.8%
[0047] Polyvinylpyrrolidone K90 3%
[0048] Dry accessories:
[0049] Magnesium stearate 1.4%
[0050] Talc 1.8%
[0051] 1000 pieces in total
[0052] The above-mentioned hypromellose K100 and glyceryl behenate are slow-release agents, lactose is a filler, talc and magnesium stearate are lubricants, and polyvinylpyrrolidone K90 in ethanol is a binder.
[0053] Preparation:
[0054] Prepare the materials according to the componen...
Embodiment 2
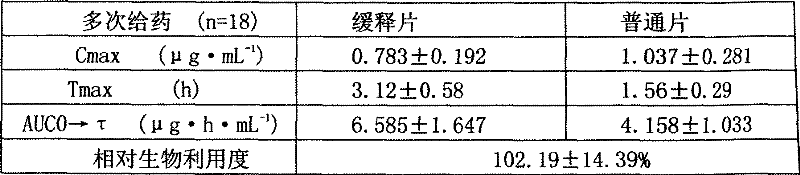
[0056] The metformin hydrochloride sustained-release tablet includes a main drug, a sustained-release material and other auxiliary materials, and is characterized in that it is formulated in the following weight percentages: 68% of the main drug, 18% of the sustained-release material, and 14% of other auxiliary materials.
[0057] The sustained-release tablet is prepared from the following raw materials in weight ratio:
[0058] 1000 tablets prescription
[0059] Metformin hydrochloride 68%
[0060] Hypromellose K100 12%
[0061] Glyceryl behenate 6%
[0062] Lactose 7%
[0063] Polyvinylpyrrolidone K90 3.8%
[0064] Dry accessories:
[0065] Magnesium stearate 1.4%
[0066] Talc 1.8%
[0067] 1000 pieces in total
[0068] The above-mentioned hypromellose K100 and glyceryl behenate are slow-release agents, lactose is a filler, talc and magnesium stearate are lubricants, and polyvinylpyrrolidone K90 in ethanol is a binder.
[0069] Preparation:
[0070] Prepare the materials according to the comp...
Embodiment 3
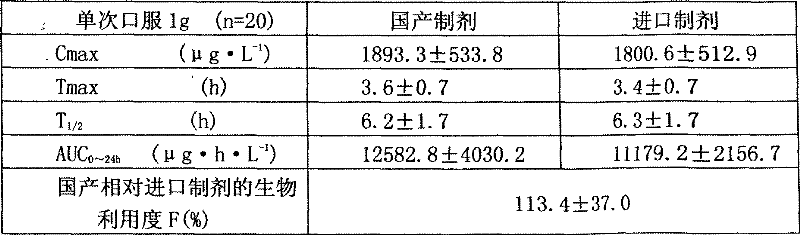
[0072] The metformin hydrochloride sustained-release tablet includes a main drug, a sustained-release material and other auxiliary materials, and is characterized by being formulated in the following weight percentages: 68% of the main drug, 22% of the sustained-release material, and 10% of other auxiliary materials.
[0073] The sustained-release tablet of the present invention is prepared from the following raw materials with a weight ratio:
[0074] 1000 tablets prescription
[0075] Metformin hydrochloride 68%
[0076] Hypromellose K100 20%
[0077] Glyceryl behenate 2%
[0078] Lactose 4.8%
[0079] Polyvinylpyrrolidone K90 2%
[0080] Dry accessories:
[0081] Magnesium stearate 1.4%
[0082] Talc 1.8%
[0083] 1000 pieces in total
[0084] Among them, hypromellose K100 and glyceryl behenate are slow-release agents, lactose is a filler, talc and magnesium stearate are lubricants, and polyvinylpyrrolidone K90 in ethanol is a binder.
[0085] Preparation:
[0086] Prepare the materials accor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com