Electrochemistry of carbon subfluorides
An electrochemical, low-fluorinated coke technology, applied in the field of bulk, can solve problems such as poor conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Embodiment 1: the synthesis of subfluorinated graphite
[0113] Graphite (the natural graphite of 7.5 μm of Madagascar (Madagascar) or the artificial graphite of 15-20 μm that the amount M is 10-30 grams is distributed on the nickel plate, 0.2g / cm 2 ) into a nickel reactor (4 L) and dried under vacuum at room temperature for 2 hours. Then, fluorine gas (purity 99.90%) was introduced up to 1 atm, after which fluorine gas was inflowed at a controlled rate (FL g / hour) using an open system reactor. Afterwards, the temperature was increased regularly (1° C. / min) to a final temperature T (° C.) and held for H hours. After the reaction, the reactor was cooled to room temperature. Next, excess fluorine was evacuated for 3 hours under a flow of dry nitrogen. (Note: F / C molar ratio of subfluorinated graphite is measured by gravimetric uptake method.)
[0114] Synthesis of CF0.63
[0116] Fluorine flow rate FL=2g / hour
[0117] Reaction time H=...
Embodiment 2
[0149] Embodiment 2: Characterization of subfluorinated graphite
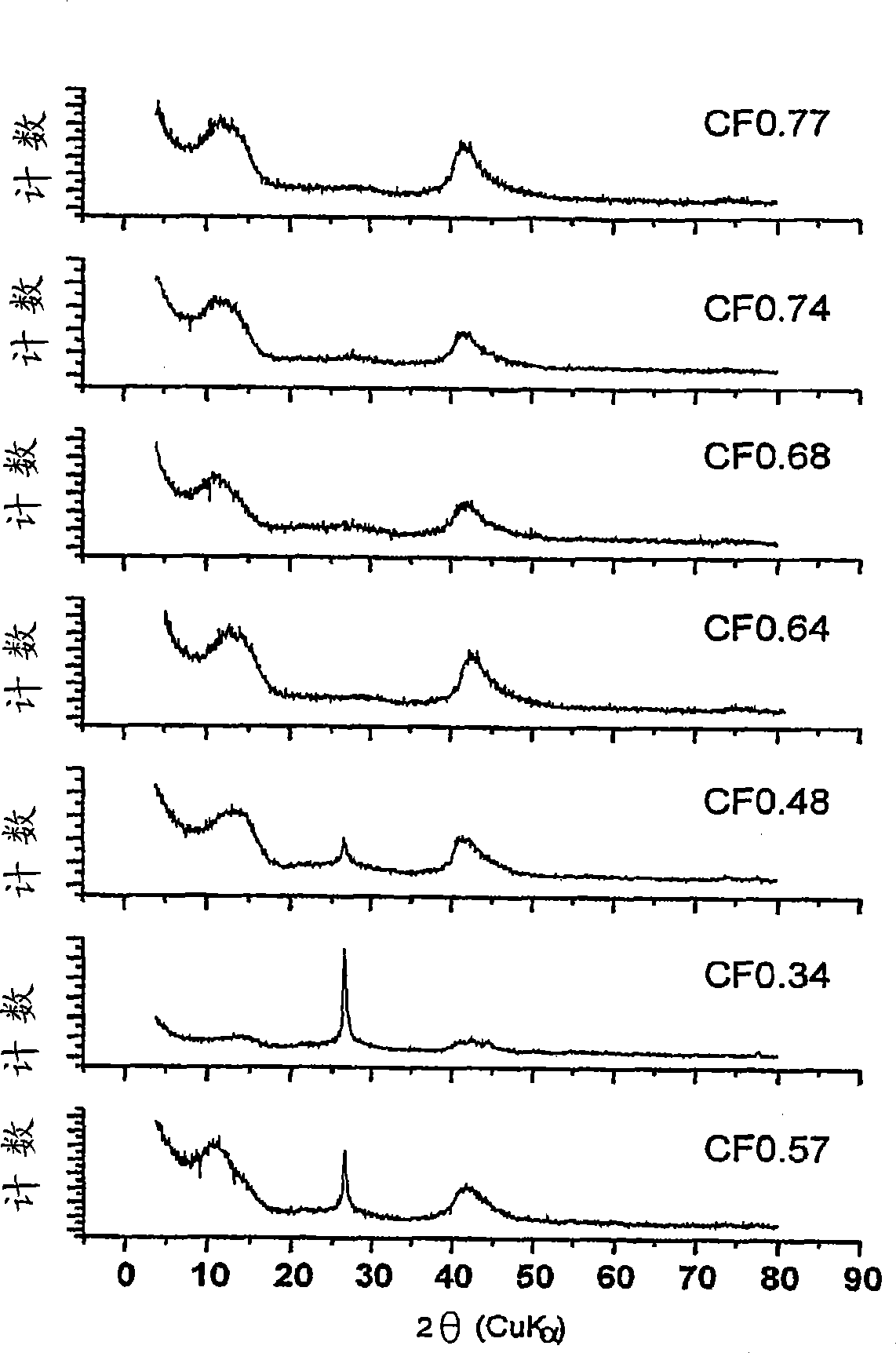
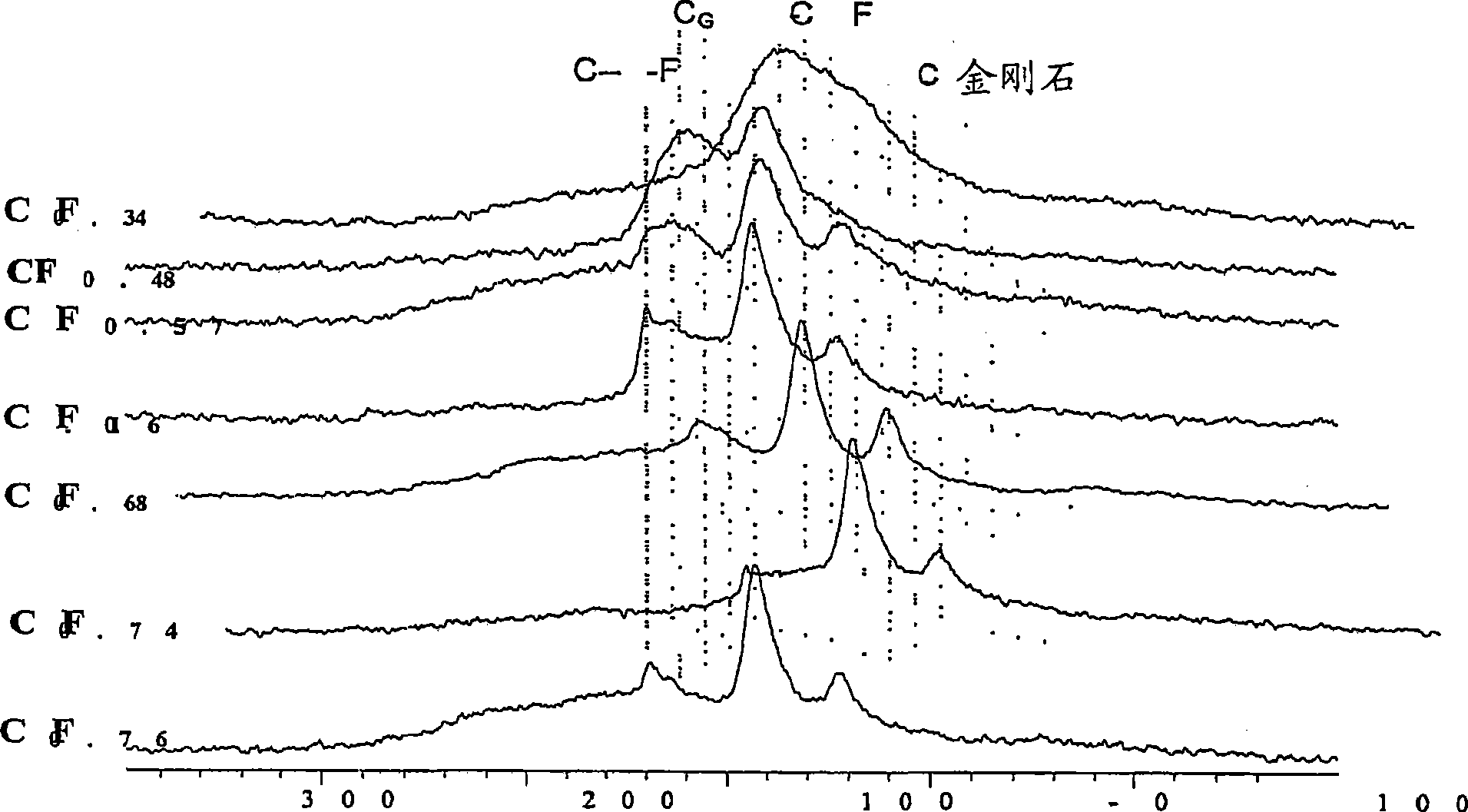
[0150] figure 1 X-ray diffraction spectra of subfluorinated graphitic materials with a range of F / C ratios are demonstrated. The spectrum exhibits peaks characteristic of a fluorinated graphite matrix between about 10 and 15 degrees 2Θ and between about 40 and 45 degrees. At 2θ = 26.3, it is clearly observed that CF 0.34 、CF 0.48 and CF 0.57 The peak; this place is the characteristic position of (002) graphite peak. The X-ray diffraction (XRD) powder diffraction pattern was obtained using a diffractometer with Cu(K α )radiation( ) and obtained. The spectrum is a plot of intensity (counts) versus 2Θ.
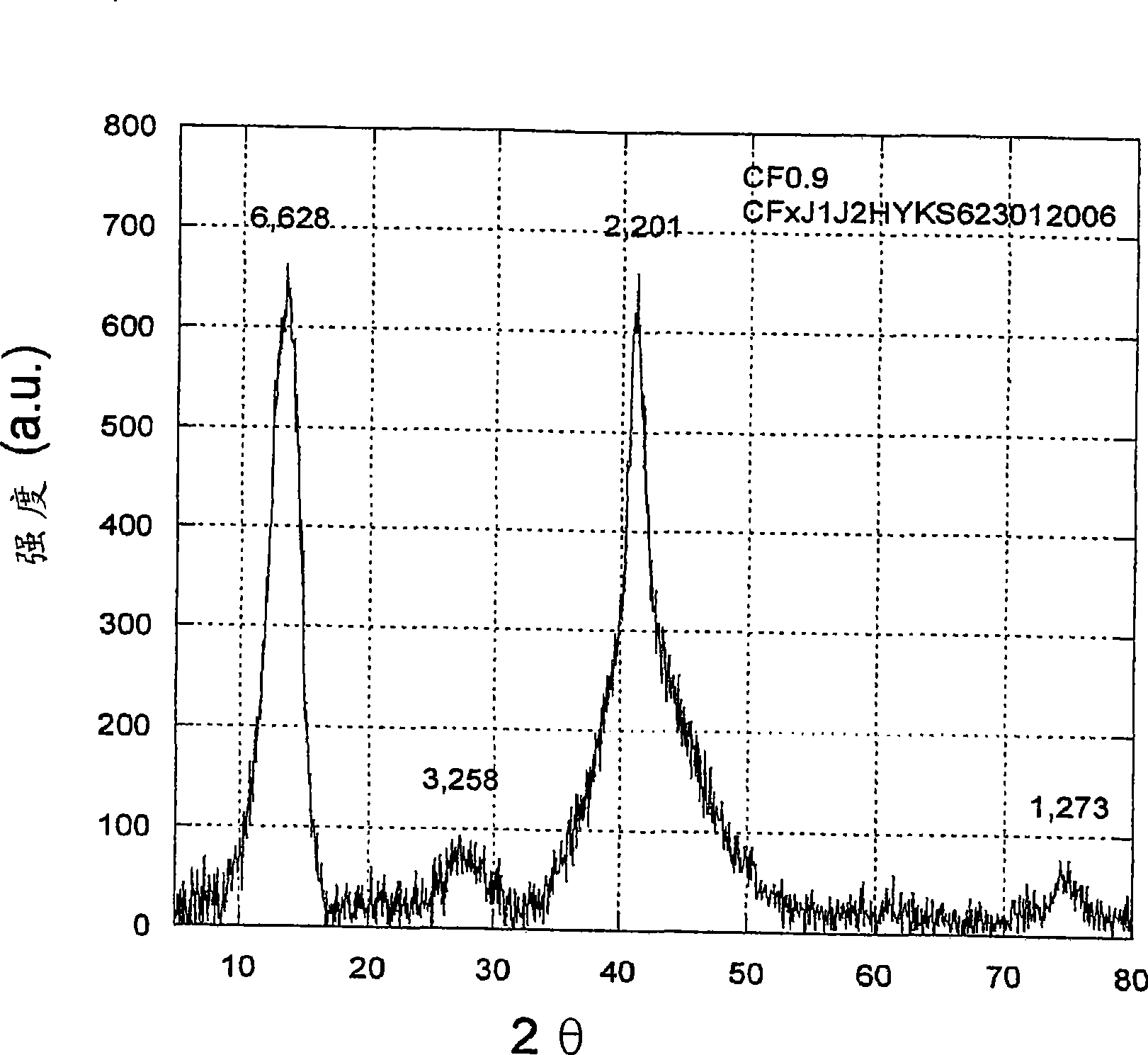
[0151] figure 2 A zoomed-in view of the X-ray diffraction spectrum of a subfluorinated graphite material with an F / C ratio of 0.9 is shown. The spectrum exhibits characteristic peaks at about 13 degrees (002) and about 41 degrees (100) of 2Θ for the fluorinated graphite matrix. The other peaks are loc...
Embodiment 3
[0157] Embodiment 3: the electrochemical characteristic of subfluorinated graphite
[0158] For electrochemical experiments, the positive electrode consists of subfluorinated carbonaceous materials, conductive materials, and binders. The positive electrode was then installed in a two-electrode cell in which the electrolyte consisted of 1 mol.L dissolved in propylene carbonate (PC) and dimethyl ether (DME). -1 LiBF 4 Solution composition. A microporous polyethylene or polypropylene film containing the electrolyte is inserted between the fluorinated graphite electrode and the lithium metal foil.
[0159] Figure 6 A Ragone plot of energy density versus the square root of power density is shown. Low fluorinated graphite CF 0.90 、CF 0.77 and CF 0.744 , compared with CF, when the power density is greater than 625W / kg, it has significantly higher energy density.
[0160] Figure 7-10 Graphite Subfluoride CF 0.9 、CF 0.77 、CF 0.744 and CF 0.647 discharge curve. The elect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Average size | aaaaa | aaaaa |
| Energy density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com