Method for electrowinning copper nitrate solution
A technology of copper nitrate and solution, which is applied to the improvement of process efficiency, photography technology, instruments, etc., can solve the problems of remelting, difficult to adopt, and no successful research reports, etc., and achieve the effect of low cost and short path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
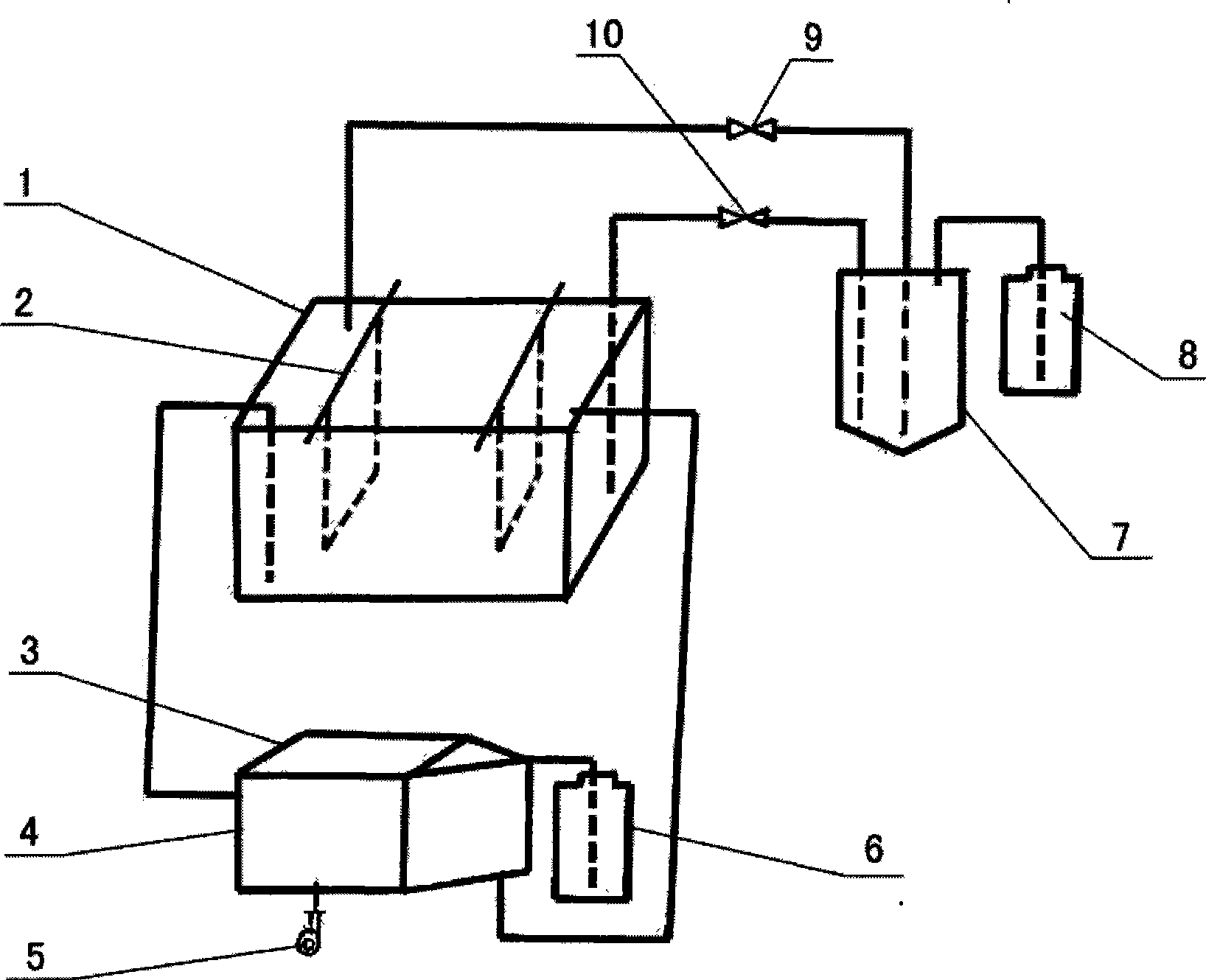
[0024] with attached figure 1 The shown equipment handles copper nitrate solution and operates as follows:
[0025] ①Use a titanium-coated electrode plate as the insoluble anode, and use a stainless steel plate as the cathode. Electrolyzer 1 is installed.
[0026] ② Concentrate the copper nitrate solution to 120 g liters and add it to the electrolytic cell 1.
[0027] ③Start work. Electrowinning conditions: temperature: ≤32°C, current density 200A / h.
[0028] ④ Take out the copper nitrate solution at one end of the electrolytic cell 1, introduce it into the gas blowing tank 4, and then introduce the copper nitrate solution from the gas blowing tank 4 to the other end of the electrolytic cell 1 to form a cycle. The rate of circulation is 8 times the total amount of electrolyte per hour. The blower 5 blows air into the blowing pool, and the blowing air volume is 50 times the volume of the electrolyte per hour. A nitric acid collector 3 is arranged above the blowing tank to...
Embodiment 2
[0034] with attached figure 1 The shown equipment handles copper nitrate solution and operates as follows:
[0035] ①Use a titanium-coated anode as the insoluble anode, and use a stainless steel plate as the cathode. Electrolyzer 1 is installed.
[0036] ② Concentrate the copper nitrate solution to 120 g liters and add it to the electrolytic cell 1.
[0037] ③Start work. Electrowinning conditions: temperature: ≤32°C, current density 250A / h.
[0038] ④ Take out the copper nitrate solution from one end of the electrolytic cell 1, introduce it into the gas blowing tank 4, and then introduce the copper nitrate solution from the gas blowing tank 4 to the other end of the electrolytic cell 1 to form a cycle. The rate of circulation is 4 times the total amount of electrolyte per hour. Air is blown into the blowing tank 4 by the blower 5, and the blowing air volume is 5 times of the electrolyte volume per hour. A nitric acid collector 3 is arranged above the blowing tank 4 to co...
Embodiment 3
[0044] with attached figure 1 The shown equipment handles copper nitrate solution and operates as follows:
[0045] ①Use a titanium-coated anode as the insoluble anode, and use a stainless steel plate as the cathode. Electrolyzer 1 is installed.
[0046] ② Concentrate the copper nitrate solution to 120 g liters and add it to the electrolytic cell.
[0047] ③Start work. Electrowinning conditions: temperature: ≤32°C, current density 300A / h.
[0048] ④ Take out the copper nitrate solution at one end of the electrolytic cell 1, introduce it into the gas blowing tank 4, and then introduce the copper nitrate solution from the gas blowing tank 4 to the other end of the electrolytic cell 1 to form a cycle. The rate of circulation is 0.5 times the total amount of electrolyte per hour. Air is blown into the blowing pool 4 by the blower 5, and the blowing air volume is 30 times the volume of the electrolyte per hour. A nitric acid collector 3 is arranged above the blowing tank 4 to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com