Method for preparing potassium fluoborate
A technology of potassium fluoroborate and boric acid, which is applied in the field of preparation of potassium fluoroborate and potassium fluoroborate, can solve the problems of lack of market competitiveness of potassium fluoroborate, low added value and high cost of anhydrous hydrofluoric acid, and achieve good economy Benefits, reduced production costs, and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
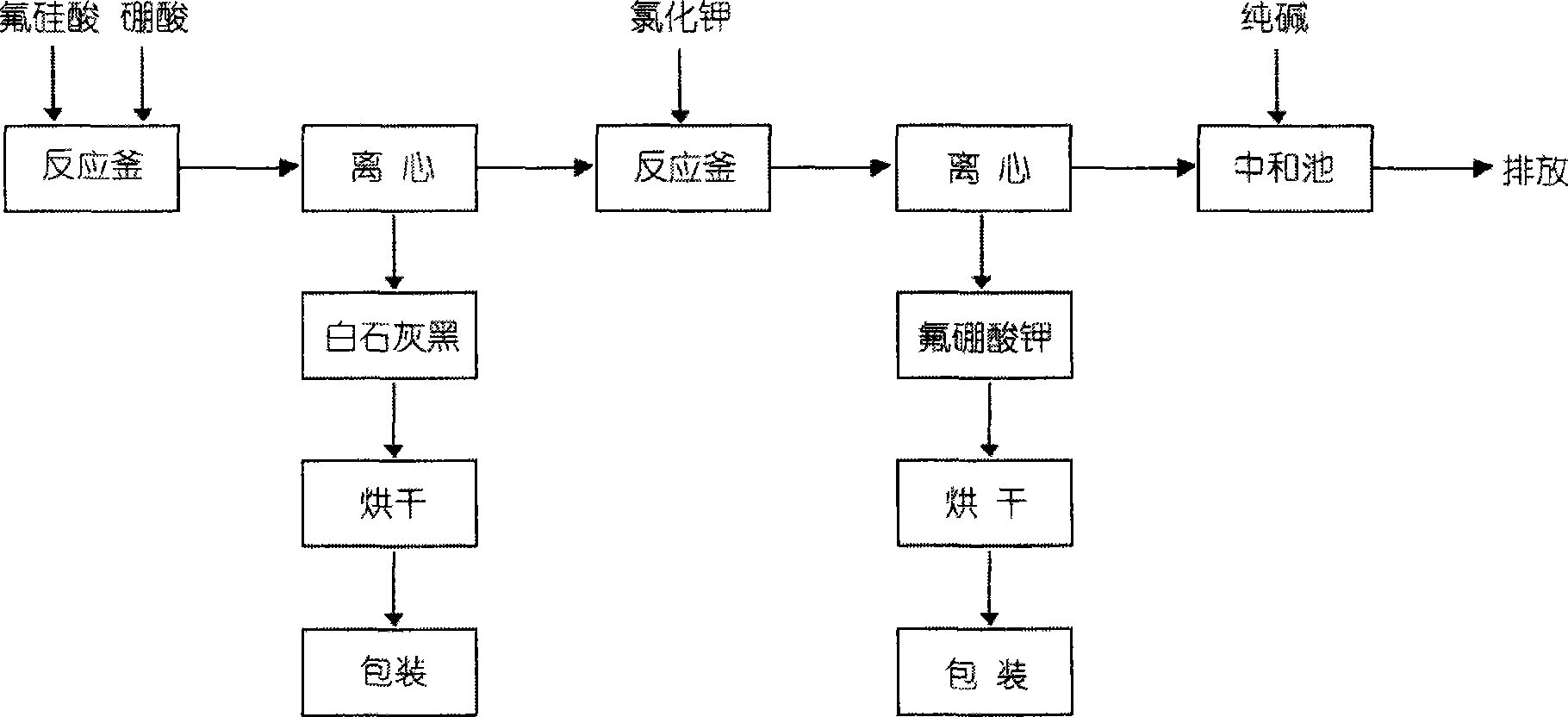
[0024] According to HG / T2832-1997, the content of fluosilicic acid in the waste fluosilicic acid (Zhejiang Zhongying Group Co., Ltd.) is 34.2%, and the content of hydrofluoric acid is 8.6%. Then weigh 150g of fluosilicic acid, add 43.5g of boric acid, stir and heat in a water bath , the temperature was controlled at 60°C for two hours, filtered and washed until neutral to obtain SiO 2 85.4g. Weigh 57g of potassium chloride and dissolve it in 140g of water, and heat until it is completely dissolved, then add it into the mother liquor, stir, and control the temperature at 60°C. Bake in a circulating oven for 4 hours, weigh 76.26g, the content is 98.16%, and the yield is 87.0%.
Embodiment 2
[0026] Through HG / T2832-1997, the content of fluosilicic acid in waste fluosilicic acid (Zhejiang Zhongying Group Co., Ltd.) is 41.5%, and the content of fluoboric acid is 0.5%. Then, 150g of fluosilicic acid is weighed, and 41g of boric acid is added, stirred and heated in a water bath. Control 30°C, react for 2 hours, suction filter and wash until neutral to obtain SiO 2 103.6g, weigh 54g of potassium chloride, dissolve it in 130g of water, heat it to dissolve completely, add it to the mother liquor, control the temperature at 95°C, after the reaction is complete, filter with suction and wash until neutral to obtain 76.15g of potassium fluoroborate, put it in an oven at 100°C Middle baked for 4 hours, weighed 70.8g, the content was 98.06%, and the yield was 85.6%.
Embodiment 3
[0028] According to MG / T 2832-1997, the content of fluosilicic acid in fluosilicic acid (Zhejiang Zhongying Group Co., Ltd.) is 48.2%. The content of hydrofluoric acid is 1.3%, then weigh 150g of fluosilicic acid, add 48.2g of boric acid, stir and heat in a water bath, control the temperature at 95°C, react for 2 hours, filter and wash to obtain neutral SiO 2 120g, about 64g of potassium chloride was dissolved in 160g of water. Heat it until it is completely dissolved and add it to the mother liquor. The temperature was controlled at 30°C. After the reaction was completed, 90g of potassium fluoroborate was obtained by suction filtration and washing to neutrality, and 83.7g was weighed in a circulating oven at 100°C for 4 hours, the content was 98.12%, and the yield was 85.6%.
[0029] After the above process, the fluorine and silicon in the waste fluosilicic acid of hydrofluoric acid manufacturers are converted into potassium fluoborate and silicon dioxide with higher added ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com