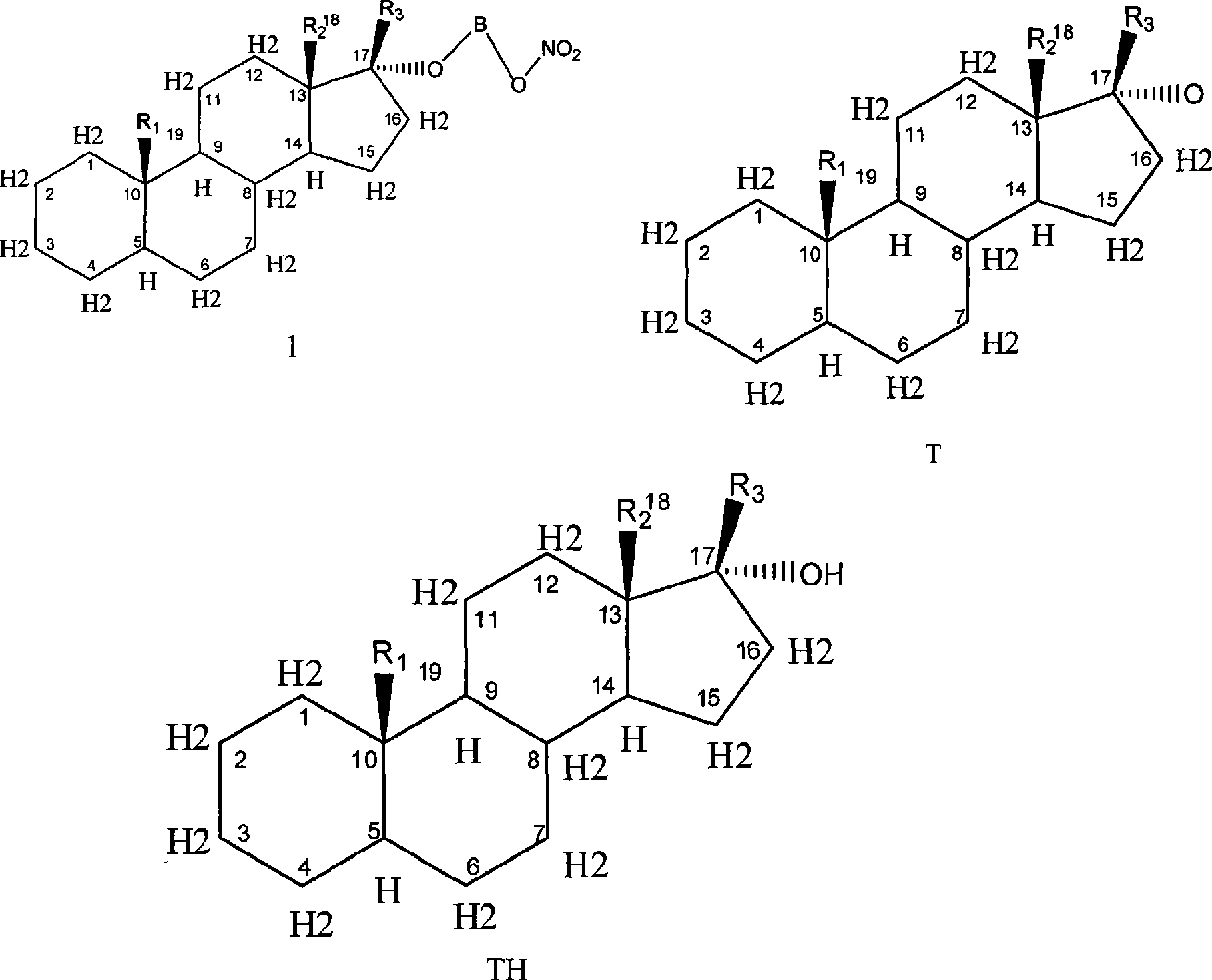
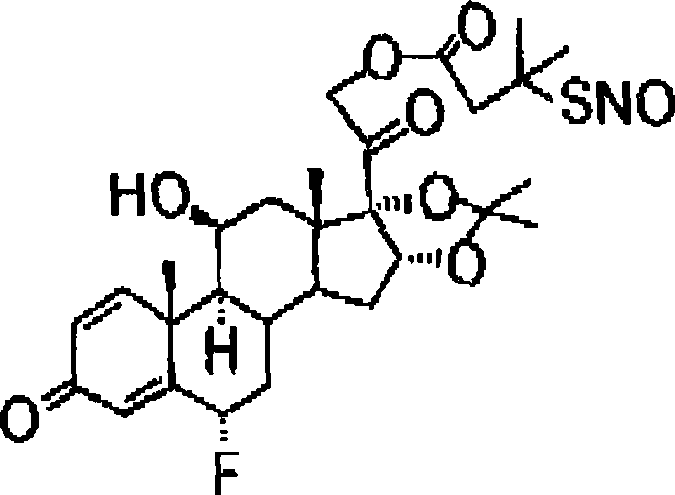
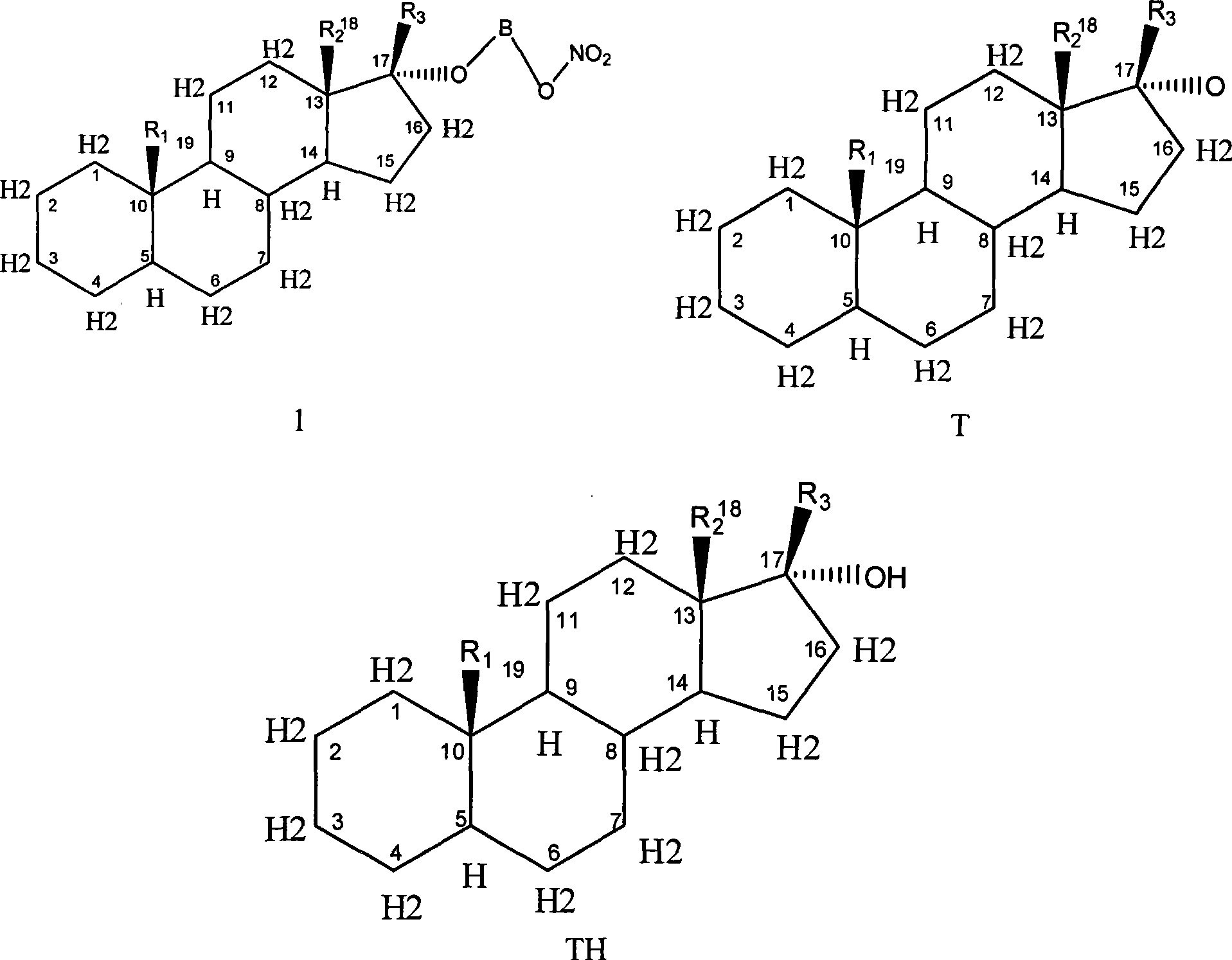
Novel nitric acid esters steroidal compounds
A compound and hydrocarbon technology, applied in the directions of steroids, non-active components of polymer compounds, organic chemistry, etc., can solve problems such as loss of ulcers, and achieve the effects of low side effects, high activity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1. methylprednisolone acetate 17-(3-nitrooxy) propionate
[0098]
[0099] 1 Methylprednisolone acetate 17-(3-chloro)propionate
[0100]
[0101] method 1:
[0102] 40ml of triethylamine, 0.05g of DMAP and 0.015mol of 3-chloropropionyl chloride were stirred in the reaction and cooled to 0°C, slowly added 0.01mol of methylprednisolone acetate and kept at a temperature of 0-5°C, The reaction is an exothermic reaction, so the addition rate and temperature need to be carefully controlled. Keep the temperature of the above reaction solution below 10°C. After the addition is completed, it will slowly rise to 10-15°C within 1 hour. , diluted to 50ml, the pH is 1-2, the temperature is 0 degrees water, slowly add hydrochloric acid to adjust the pH to 3-5, the liquid is extracted three times with 90ml of dichloromethane (30mlX3), and then washed with 90ml of water three times (30mlX3) Adjust the pH value of the dichloromethane layer to neutral, concentrate the ...
Embodiment 2
[0112] Example 2. Methylprednisolone 17-(3-nitrooxy)propionate
[0113]
[0114] 0.002mol of methylprednisolone acetate 17-(3-nitrooxy)propionate prepared as above was dissolved in 20ml of methanol and chloroform (1:1), and added dropwise at 0°C under nitrogen protection. Saturated 0.005 mol aqueous sodium carbonate solution was vigorously stirred for 10 hours, the pH of the reaction system was adjusted to neutral with hydrochloric acid, concentrated under reduced pressure, chloroform was removed, the solution was diluted in 50 ml of ice water, filtered, and dried to obtain the crude product of the title compound. The crude product was subjected to column chromatography and eluted with methanol and chloroform (1:4) as the mobile phase. The main compound was concentrated under reduced pressure, washed into methanol for recrystallization, and 0.001 mol of the title compound was obtained.
[0115] Elemental analysis calculated values: C 61.09%, H 6.77%, N 2.85%, O 29.30%,
[...
Embodiment 3
[0119] Embodiment 3. Betamethasone acetate 17-(5-nitrooxy)valerate
[0120]
[0121] Taking betamethasone acetate as raw material, according to the method of embodiment 1 and 5-bromovaleryl chloride, AgNO 3 The reaction affords the title compound.
[0122] Elemental analysis calculated value (%): C, 60.09; H, 6.61; F, 3.28; N, 2.42; O, 27.60
[0123] Elemental analysis measured value: C29H38FNO10 C 60.00%, H 6.63%, N 2.33%, F 3.34 O 27.70%
[0124] 13 C-NMR (CDCl 3 ): 1 to 29 carbon values:
[0125] 152.0, 128.5, 185.9, 121.4, 166.7, 31.2, 27.1, 34.1, 100.9, 47.9, 70.9, 37.2, 46.8, 44.0, 33.2, 47.2, 94.8, 17.1, 22.1, 212.5, 68.4, 174.1, 34.2, 21. 74.9, 170.1, 20.9, 19.81
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com