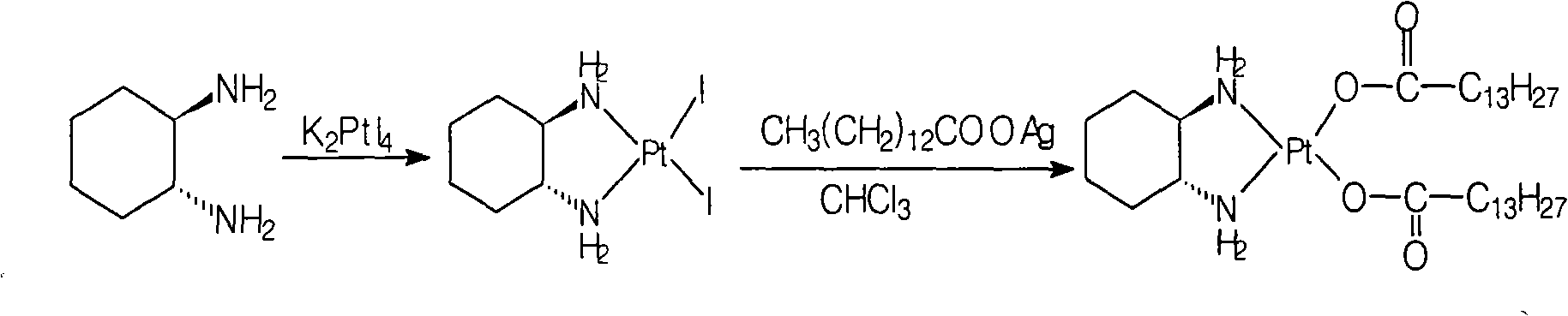Process for producing platinum
A technology of miplatin and cis, which is applied in the field of medicinal chemistry, can solve the problems of production personnel and environmental impact, and achieve the effects of low production cost, convenient product separation, and reduced treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
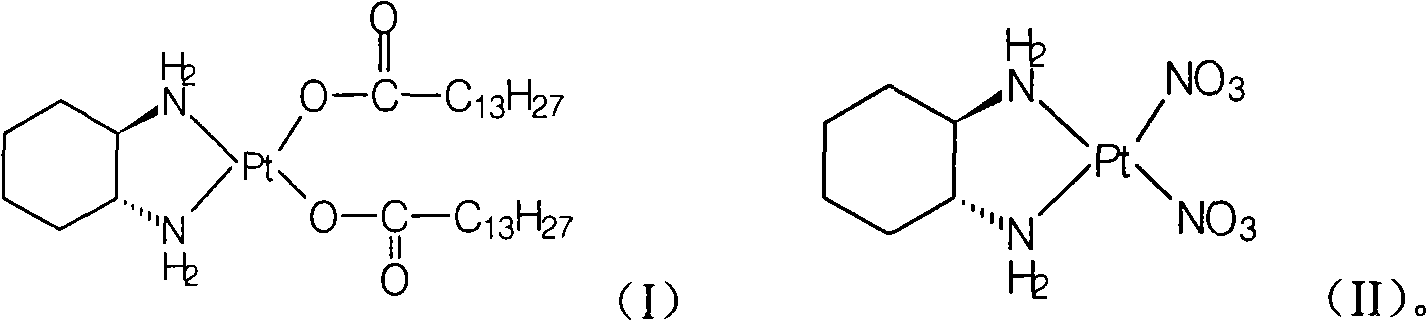
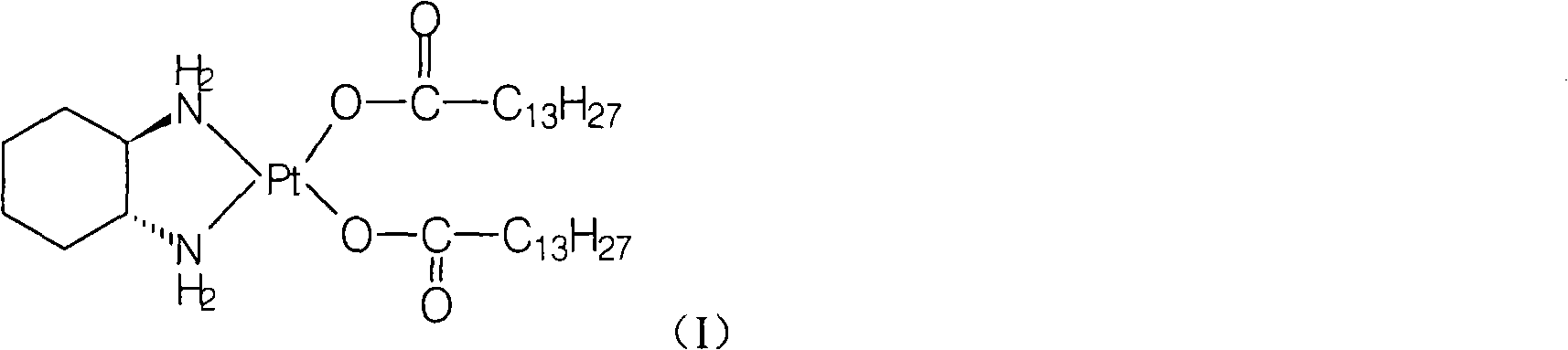
[0020] Preparation of Miplatin:
[0021] Add 5.2g of cis-dinitrate [(1R,2R)-1,2-cyclohexanediamine] platinum(II) (0.012mol) and 100ml of water into a 250ml reaction flask, stir, heat to 50°C, and keep warm Add 6.3 g (0.025 mol) of sodium myristate until the solid is dissolved and clear, and react at 50°C for 2 hours, cool to room temperature, filter the precipitated solid, wash the filter cake with water, and dry under vacuum at 40°C to obtain the target product (monohydrate) 8.3 g, yield: 88.4%.
[0022] Chemical purity: 99.28%. (HPLC conditions: use octadecylsilane bonded silica gel as filler; mobile phase is phosphate buffer [1.36g potassium dihydrogen phosphate is added in 10ml tetrabutylammonium hydroxide solution (32%), add water and dilute to 1000ml, use Adjust the pH value to 6.0 with phosphoric acid]-methanol (70:30); the flow rate is 1.0ml / min; the detection wavelength is 216nm.)
[0023] Optical purity: 100%. (HPLC condition: adopt chiral chromatographic column ...
Embodiment 2
[0029] The preparation of cis-dinitrate [(1R, 2R)-1,2-cyclohexanediamine] platinum (II):
[0030] Refer to the methods disclosed in documents such as patent EP567438 or EP617043:
[0031] Add cis[((1R,2R)-1,2-cyclohexanediamine-dichloro]platinum 25g (0.066mol) and water 500ml into a 1L reaction flask, stir, add silver nitrate 22.3g (0.13mol), Stir the reaction for 24 hours, remove the silver chloride solid by filtration, remove most of the water by distillation under reduced pressure at 50°C (ethanol with water can be used), cool to room temperature, filter the precipitated solid, wash the filter cake with water, and dry under vacuum at 40°C to obtain the white target product 25.9 g, yield: 90.6%.
Embodiment 3
[0033] Preparation of Miplatin:
[0034] Add 2.5g of cis-dinitrate [(1R,2R)-1,2-cyclohexanediamine] platinum(II) (5.8mmol) and 125ml of water into a 500ml reaction bottle, stir until the solid is dissolved and clarified, add meat Sodium myristate 2.9g (11.6mmol), react at room temperature for 7 hours, filter the precipitated solid, wash the filter cake with water, and vacuum dry at 40°C to obtain 3.5g of the target compound (monohydrate), yield: 77.2%. Chemical purity: 99.27%, optical purity: 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com