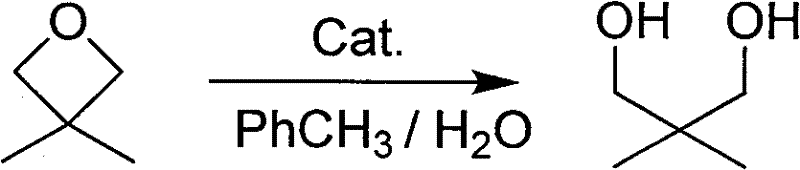Efficient recovery method for neopentyl glycol
A technology of neopentyl glycol and recovery method, which is applied in the production of bulk chemicals, hydrolysis preparation, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Feed mass ratio 3,3-dimethyl propylene oxide: phosphoric acid is 100:1 feed intake, hydrolysis toluene 100g (wherein 3, the content of 3-dimethyl propylene oxide is 5g); Phosphoric acid is 0.05g; Solvent water 100g .
[0026] 0.05g of phosphoric acid was dissolved in 100g of water, and 100g (5wt%) of hydrolyzed toluene was added dropwise. After the addition was completed, the reaction was carried out at 95° C., and the reaction was complete in 15 hours.
[0027] After the reaction was completed, the temperature of the water bath was cooled to room temperature, and the layers were separated. The water layer was taken, and the pH value was adjusted to neutrality with 40% sodium hydroxide solution. The water was distilled off under reduced pressure. 150g, heated to reflux, dewatered by the water separator, after 2h the water was removed, filtered while it was hot, removed a little insoluble matter in the reaction bottle, took the filtrate and transferred it to a flask, sti...
Embodiment 2
[0029] Feed mass ratio 3,3-dimethyl propylene oxide: phosphoric acid is 87:1 feed intake, hydrolysis toluene 100g (wherein 3, the content of 3-dimethyl propylene oxide is 20g); Phosphoric acid is 0.23g; Solvent water 100g .
[0030] The reaction temperature was 91° C., the reaction time was 10 hours, the amount of toluene added was 100 g, and the water separator’s water removal time was 2 hours. Other operations were the same as in Example 1 to obtain 20.61 g of neopentyl glycol with a yield of 85.2% and a purity of 98.1%.
Embodiment 3
[0032] Feed mass ratio 3,3-dimethyl propylene oxide: phosphoric acid is 29.4:1 feed intake, hydrolysis toluene 100g (wherein 3, the content of 3-dimethyl propylene oxide is 10g); Phosphoric acid is 0.34g; Solvent water 100g .
[0033] The reaction temperature was 94° C., the reaction time was 10 hours, the amount of toluene added was 70 g, and the water separator dewatering time was 2.5 h. Other operations were the same as in Example 1 to obtain 10.86 g of neopentyl glycol with a yield of 89.8% and a purity of 98.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com