Monohydroxy-2-acyl phenylacetate, and preparation and use thereof
A kind of technology of acyl phenylacetate and hydroxyphenylpropionic acid, applied in the field of monohydroxy-2-acyl phenylacetate and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
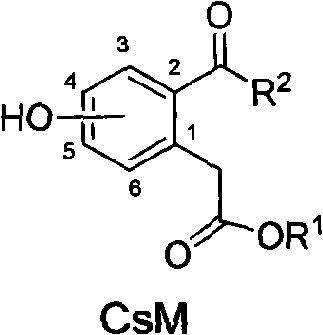
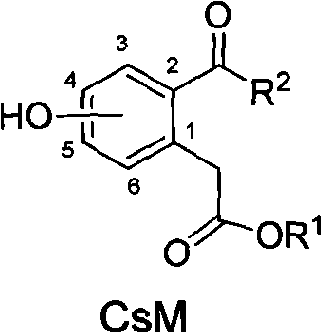
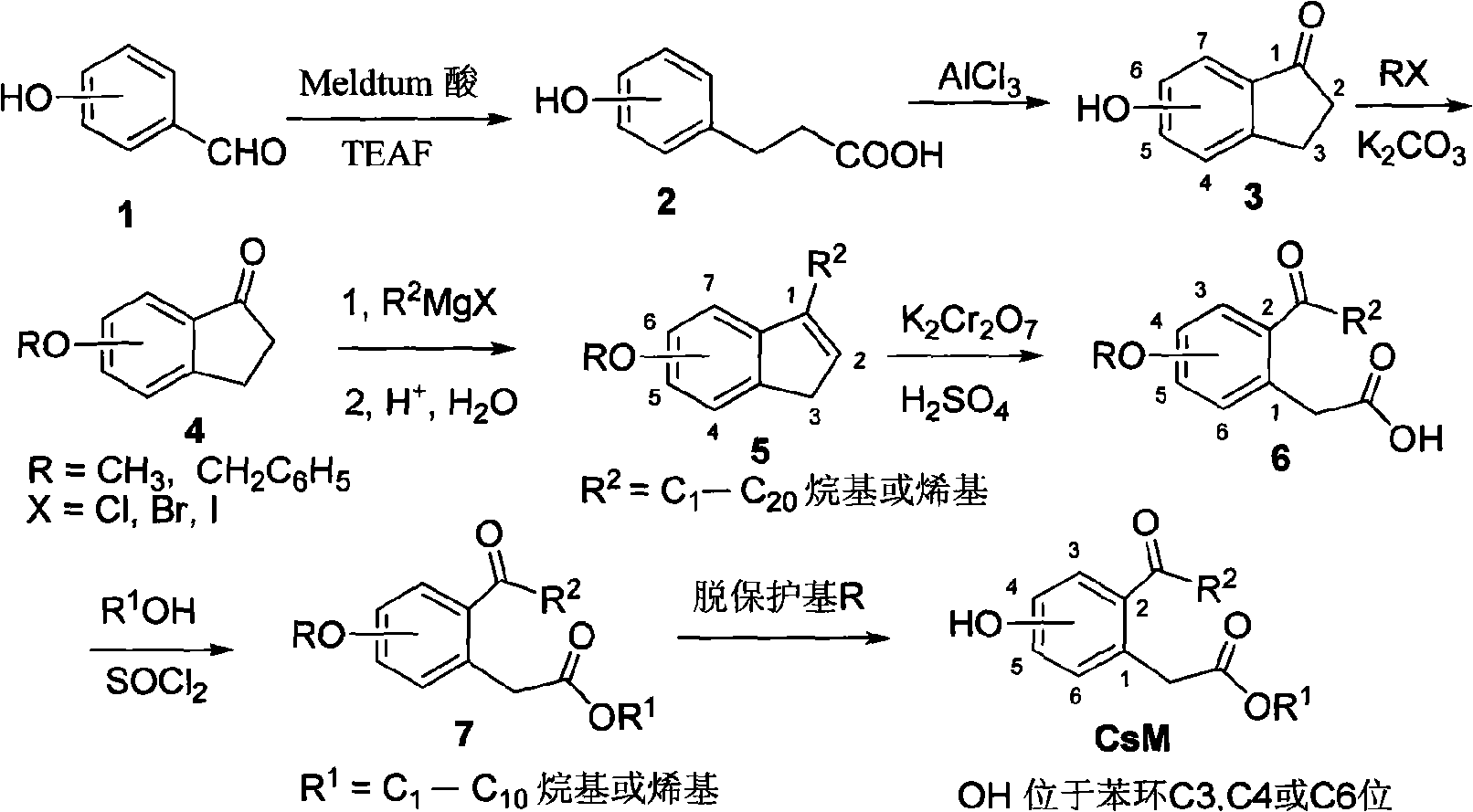
[0041] 4-Hydroxy-2-octanoylphenylacetic acid ethyl ester (compound CsM4, R 1 =C 2 h 5 , R 2 =n-C 7 h 15 )
[0042] 1) 4-hydroxyphenylpropionic acid (compound 2, OH is at the C4 position of the benzene ring)
[0043] Add triethylamine formate (TEAF ) solution (9mL), heated to 95-100°C and stirred for 3h. Carefully add ice water, adjust to pH=1-2 with 6N hydrochloric acid, extract with ethyl acetate, combine extracts, wash with saturated sodium chloride solution, anhydrous MgSO 4 After drying and concentration under reduced pressure, the residue was purified by flash column chromatography to obtain 4-hydroxyphenylpropionic acid 2 with a yield of 85%.
[0044] 2) 6-hydroxy-2,3-dihydro-1-indanone (compound 3, OH is at C6 position)
[0045] Add 4-hydroxyphenylpropionic acid (6mmol), NaCl (60mmol), anhydrous AlCl in the reaction flask 3 (24mmol), the reaction mixture was stirred at 150°C for 2h, dissolved in 2N HCl solution, extracted with dichloromethane, combined organic...
Embodiment 2
[0057] 4-Hydroxy-2-decanoyl-phenylacetic acid ethyl ester (compound CsM5, R 1 =C 2 h 5 , R 2 =n-C 9 h 19 ) The preparation of CsM5 is from embodiment 1 intermediate product 6-benzyloxy-2,3-dihydro-1-indanone (compound 4, R=CH 2 C 6 h 5 )start.
[0058] 1) 6-benzyloxy-1-nonyl-3H-indene (compound 5, R=CH 2 C 6 h 5 , R 2 =n-C 9 h 19 )
[0059] According to the method described in Preparation Method 4) in Example 1, 6-benzyloxy-2,3-dihydro-1-indanone and Grignard reagent n-C 9 h 19 The reaction with MgCl gave 6-benzyloxy-1-nonyl-3H-indene 5 with a yield of 72%.
[0060] 2) 4-benzyloxy-2-decanoyl-phenylacetic acid (compound 6, R=CH 2 C 6 h 5 , R 2 =n-C 9 h 19 )
[0061] According to the method described in preparation method 5) in Example 1, 6-benzyloxy-1-nonyl-3H-indene was oxidized with acidic potassium dichromate to obtain 4-benzyloxy-2-decanoyl-benzene Acetic acid 6, yield 45%.
[0062] 3) ethyl 4-benzyloxy-2-decanoyl-phenylacetate (compound 7, R=CH 2 ...
Embodiment 3
[0067] 4-Hydroxy-2-butyryl-pentyl phenylacetate (compound CsM6, R 1 =n-C 5 h 11 , R 2 =C 3 h 7 )
[0068] The preparation of CsM6 is from embodiment 1 intermediate product 6-benzyloxy-2,3-dihydro-1-indanone (compound 4, R=CH 2 C 6 h 5 )start.
[0069] 1) 6-benzyloxy-1-propyl-3H-indene (compound 5, R=CH 2 C 6 h 5 , R 2 =C 3 h 7 )
[0070] According to the method described in Preparation Method 4) in Example 1, 6-benzyloxy-2,3-dihydro-1-indanone and Grignard reagent C 3 h 7 MgBr reaction followed by acidic hydrolysis afforded 6-benzyloxy-1-propyl-3H-indene 5 in 78% yield.
[0071] 2) 4-benzyloxy-2-butyryl-phenylacetic acid (compound 6, R=CH 2 C 6 h 5 , R 2 =C 3 h 7 )
[0072] According to the method described in preparation method 5) in Example 1, 6-benzyloxy-1-propyl-3H-indene was oxidized with acidic potassium dichromate to obtain 4-benzyloxy-2-butyryl-benzene Acetic acid 6, yield 50%.
[0073] 3) 4-benzyloxy-2-butyryl-pentyl-phenylacetate (compound 7, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com