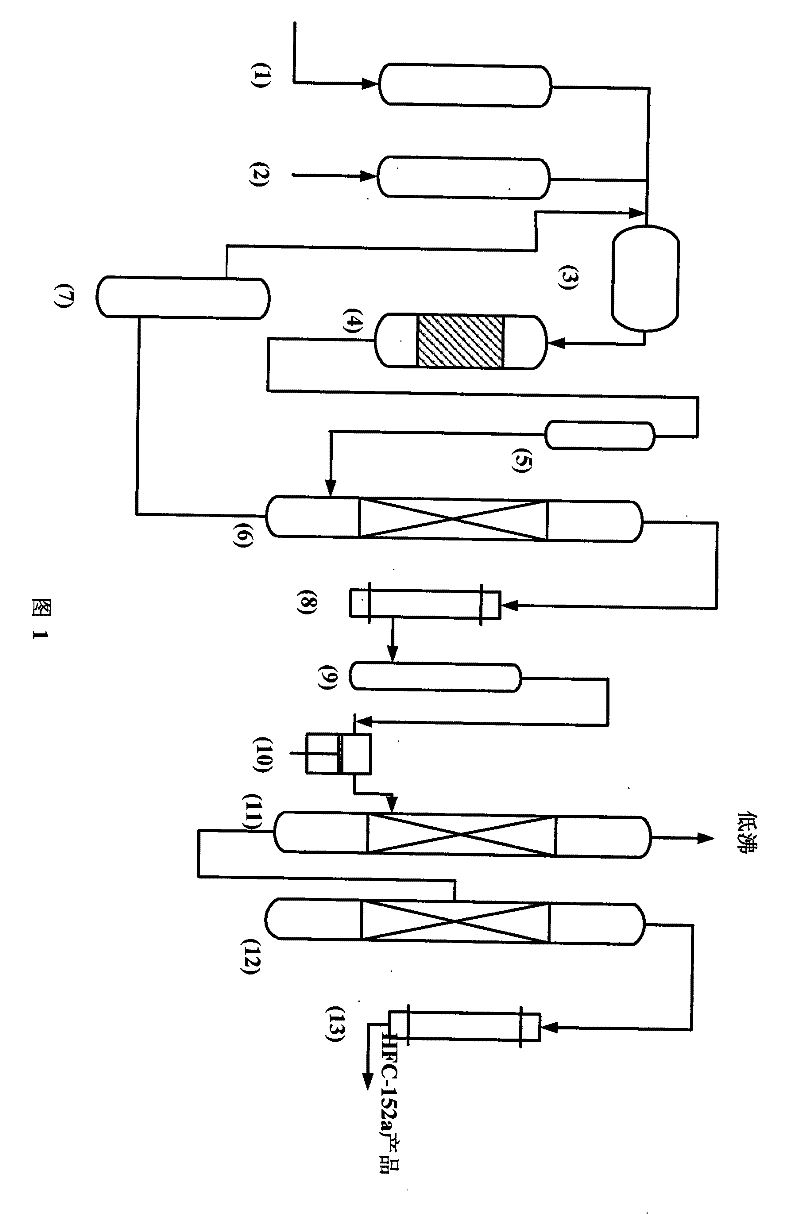
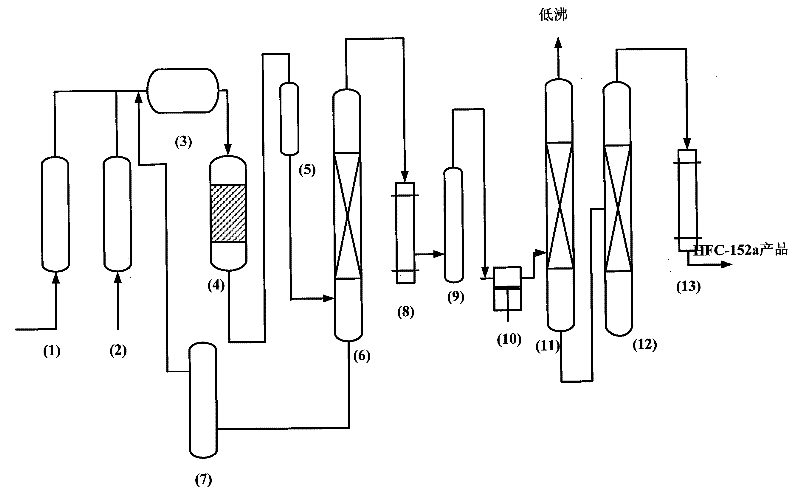
A kind of preparation method and fluorination catalyst of 1,1-difluoroethane
A technology based on fluorination catalyst and difluoroethane, applied in 1 field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] After the anhydrous hydrofluoric acid and acetylene are vaporized, they are mixed into a 180ml carbon steel fixed-bed reactor capable of receiving materials, and the fluorination reaction is carried out under the action of a chromium-based catalyst, and the material ratio of anhydrous hydrofluoric acid and acetylene is controlled 8 : 1, 230°C of reaction temperature, pressure 1.0Mpa, (under standard conditions) reactor space velocity 800h -1 , sampling and analyzing the outlet material of the reactor, the conversion rate of acetylene is 78.55%, and the selectivity of HFC-152a is 95.40%.
preparation Embodiment 1
[0052] 0.5gIn(NO 3 ) 3 9H 2 O, 4g CoCl 2 ·6H 2 O and 50g Cr(NO 3 ) 3 9H 2 O was dissolved in 800g of pure water to obtain an aqueous solution containing indium, cobalt and chromium, and ammonia water was added to the solution for precipitation reaction, so that the pH value of the reaction solution was in the range of 6.5 to 10, filtered, fully washed with distilled water, and heated at 110°C Dry for 12 hours. Grind the resulting solid, mix it with graphite, press it into tablets with a tablet press, put it into a roasting furnace, and 2 Calcined at 400° C. for 4 hours in air flow to obtain a catalyst precursor. The resulting precursor was loaded into the reaction tube, and the 2 The catalyst was activated at 350°C in a diluted AHF stream, and its physical properties are listed in Table 4.
[0053] Table 4 Physical properties of fluorination catalysts
[0054] catalyst 01 02 Bulk density (g / ml) 0.88 0.90 Specific surface area (m 2 g ...
preparation Embodiment 2
[0057] Cr(NO 3 ) 3 9H 2 The O solution was mixed with ammonia water under sufficient stirring to obtain a chromium hydroxide slurry, which was filtered, the filter cake was fully washed with distilled water, and then dried at 110° C. for 12 hours. Grind the resulting solid, and slowly drop In(NO 3 ) 3 ·nH 2 O and CoCl 2 ·6H 2 Aqueous solution of O. The powder was further dried at 110°C for 12 hours, then mixed with graphite and subsequently granulated with a granulator. Then, molding, calcining and fluorination were performed in the same manner as in Catalyst Preparation Example 1. The physical properties of the catalyst are shown in Table 5.
[0058] Table 5 Physical properties of fluorination catalysts
[0059] catalyst 03 04 Bulk density (g / ml) 0.91 0.92 Specific surface area (m 2 g -1 ) 112.8 115.6 Pore volume (ml·g -1 ) 0.35 0.34
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com