Method for preparing micropore polymer electrolyte by using glyoxaline cation-intercalated montmorillonite
An imidazolium cationic, microporous polymer technology, applied in the polymer field, can solve problems such as low thermal stability, and achieve the effects of improving electrical conductivity, convenient operation, and improving film thermal stability and flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 2 grams of dry Na-montmorillonite to 15ml of 1-ethyl-3-methylimidazolium tetrafluoroborate and 150ml of secondary aqueous solution, stir vigorously at 80°C for 6 hours, then centrifuge the hot solution, Wash twice with water (not more than 10 times) to remove halogen and tetrafluoroborate ions on the surface of montmorillonite (use 0.1mol / L silver nitrate solution to detect the presence of halogen). The obtained montmorillonite was put into an oven for vacuum drying at 100° C. for 24 hours, then ground and passed through a 325 mesh sieve to obtain the montmorillonite intercalated with imidazolium cations,
[0023] Get 0.06 gram of imidazolium cation-intercalated montmorillonite, disperse in 15 ml of N, N-dimethylformamide and ultrasonically disperse for 40 minutes to obtain a uniform solution, then add 1.5 ml of glycerol, 1.5 gram of poly Vinylidene fluoride, 1.5 grams of polyethylene glycol (molecular weight 10,000), vigorously stirred at 80°C for 6 hours to obtain...
Embodiment 2
[0026] Example 2: Add 2 grams of dry Na-montmorillonite to 15ml of 1-ethyl-3-methylimidazolium tetrafluoroborate and 150ml of secondary aqueous solution, stir vigorously at 80°C for 6 hours, and then Centrifuge the hot solution, wash with secondary water (no more than 10 times) to remove halogen and tetrafluoroborate ions on the surface of montmorillonite, and detect the presence of halogen with 0.1mol / L silver nitrate solution. The obtained modified montmorillonite was vacuum-dried at 100°C for 24 hours, then ground and passed through a 325-mesh sieve to obtain imidazolium cation-intercalated montmorillonite;
[0027] Disperse 0.12 g of imidazolium cation-intercalated montmorillonite in 15 ml of N,N-dimethylformamide and ultrasonically disperse for 40 minutes, then add 1.5 ml of glycerin, 1.5 g of polyvinylidene fluoride, and 1.5 g of polyethylene Diol (molecular weight 10,000), vigorously stirred at 80°C for 6 hours to obtain a homogeneous hot solution.
[0028] Pour the ho...
Embodiment 3
[0030] Example 3: Add 2 grams of dry Na-montmorillonite to 15ml of 1-ethyl-3-methylimidazolium tetrafluoroborate and 150ml of secondary aqueous solution, stir vigorously at 80°C for 6 hours, and then Centrifuge the hot solution, wash with secondary water (no more than 10 times) to remove halogen and tetrafluoroborate ions on the surface of montmorillonite, and detect the presence of halogen with 0.1mol / L silver nitrate solution. The obtained modified montmorillonite was vacuum-dried at 100° C. for 24 hours, then ground and passed through a 325-mesh sieve to obtain imidazolium cation-intercalated montmorillonite.
[0031] Take 0.18 g of imidazolium cation-intercalated montmorillonite and disperse it in 15 ml of N,N-dimethylformamide and ultrasonically disperse it for 40 minutes, then add 1.5 ml of glycerin, 1.5 g of polyvinylidene fluoride, and 1.5 g of polyethylene Diol (molecular weight 10000), vigorously stirred at 80°C for 6 hours to obtain a homogeneous hot solution;
[0...
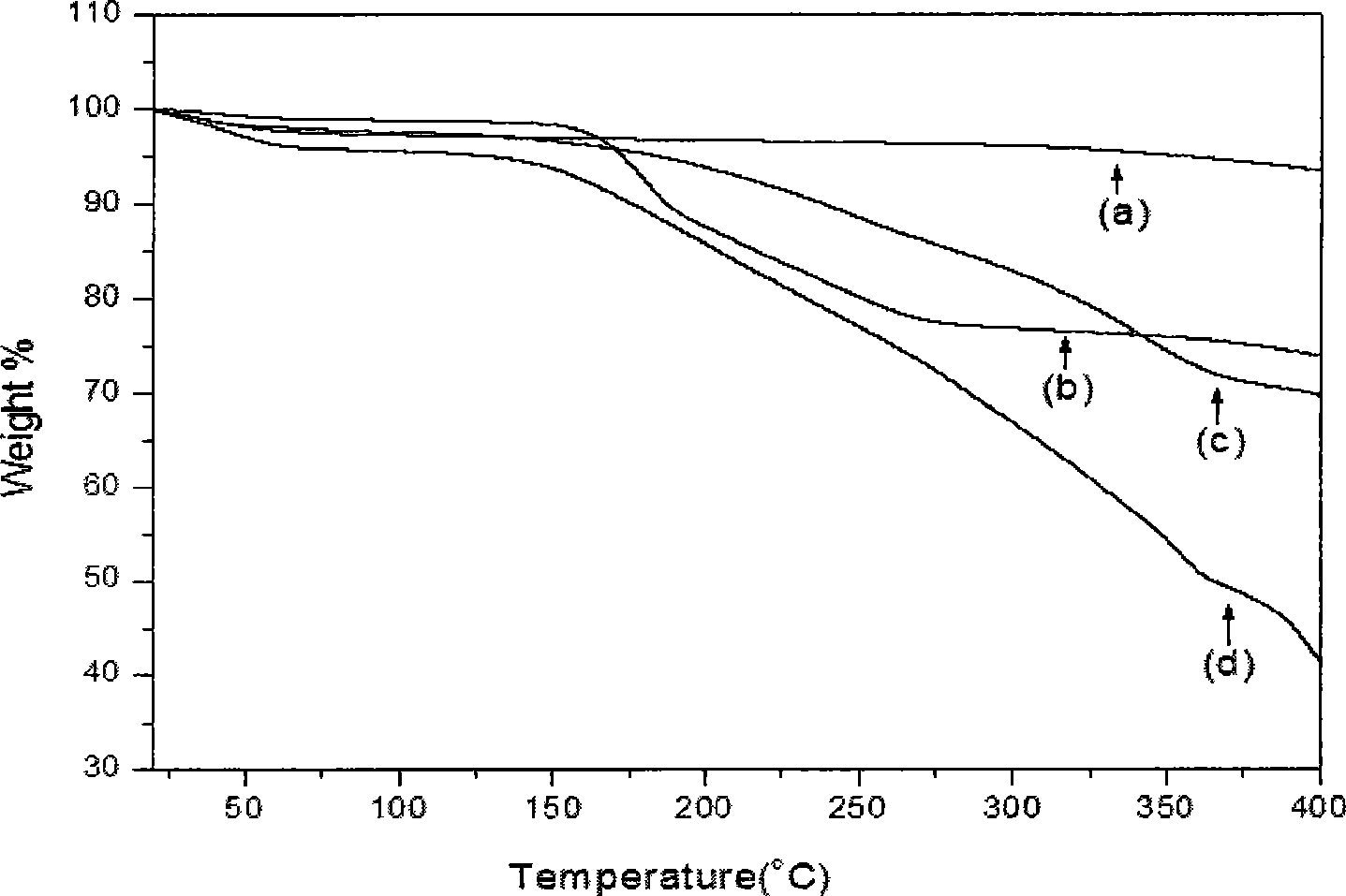
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com