Diperylene-3,4,6,7:12,13,15,16-octocarboxylic tetraimides compounds and production method thereof
A technology of octacarboxylic tetraimide and tetracarboxylic diimide, which is applied to diperylene-3,4,6,7:12,13,15,16-octacarboxylic tetraimide In the field of compounds and their preparation methods, it can solve problems such as not being seen, and achieve the effects of simple synthesis methods, novel chemical structures, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 (N, N', N", N"'-tetrakis(2,6-diisopropylphenyl)-thiabisperylene-3,4,6,7:12,13,15, 16-Octacarboxylic tetraimide and N,N',N",N"'-tetrakis(2,6-diisoendylphenyl)-dithiabiperylene-3,4,6,7 : 12,13,15,16-octacarboxylic acid tetraimide)
[0052]
[0053] Concrete synthetic steps are:
[0054] 1. Add 1mmol of N,N'-bis(2,6-diisopropylphenyl)-1,6,7,12-tetrachloroperylene-3,4:9,10-tetracarboxylic diimide , 10mmol sodium sulfide, 6mmol cuprous iodide, 7mmol L-proline and 12mmol anhydrous potassium carbonate are mixed together;
[0055] 2. Add 10ml dimethyl sulfoxide to the mixture prepared in step 1;
[0056] 3. Under nitrogen protection, heat the mixed solution prepared in step 2 to 110°C and react for 10 hours; after the reaction, the product is dissolved in ethyl acetate, washed with aqueous sodium chloride, dried over anhydrous sodium sulfate, filtered, The solvent was evaporated to dryness below 60°C, and the product N, N', N", N"'-tetrakis(2,6-diisopropylpheny...
Embodiment 2
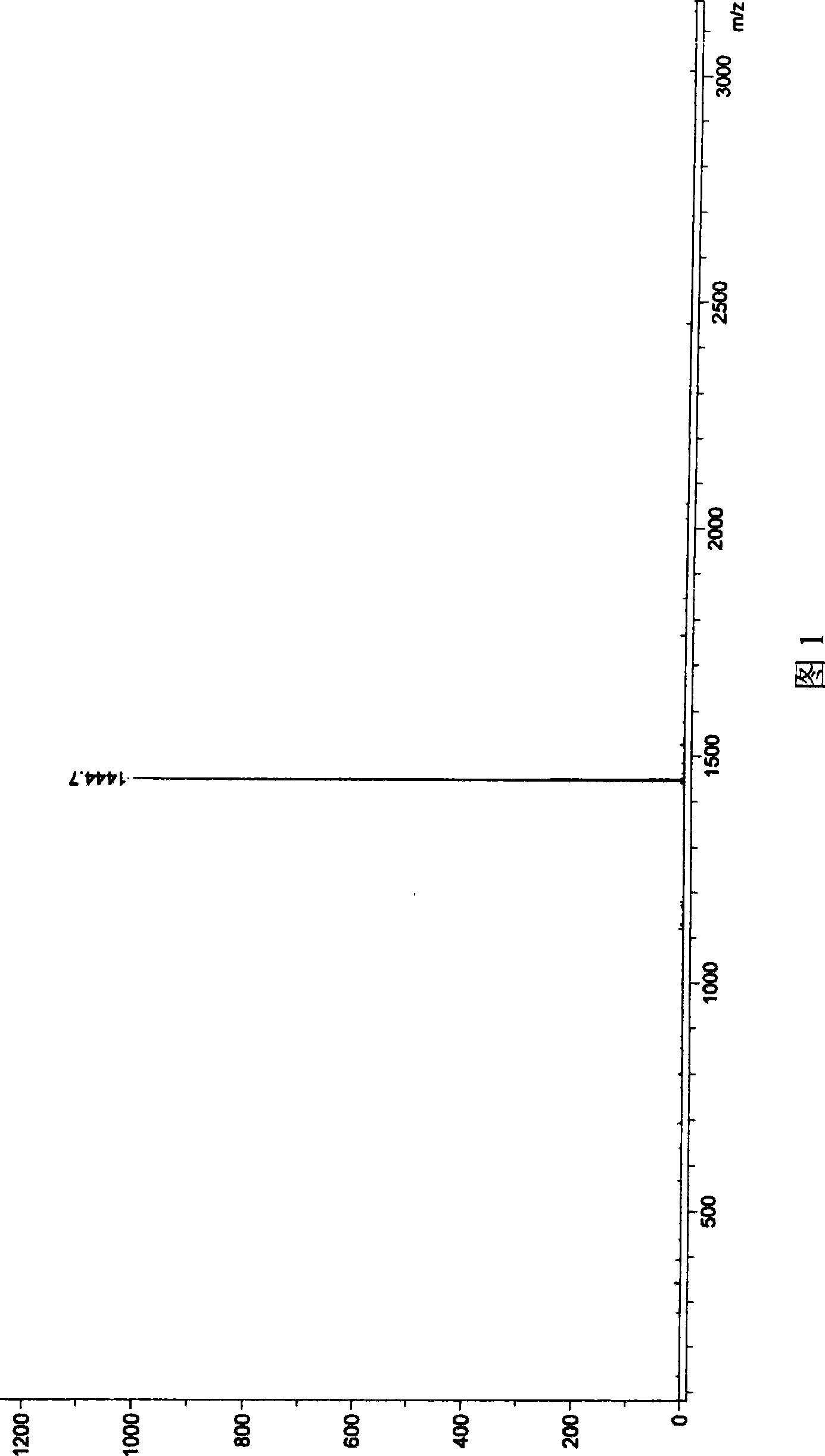
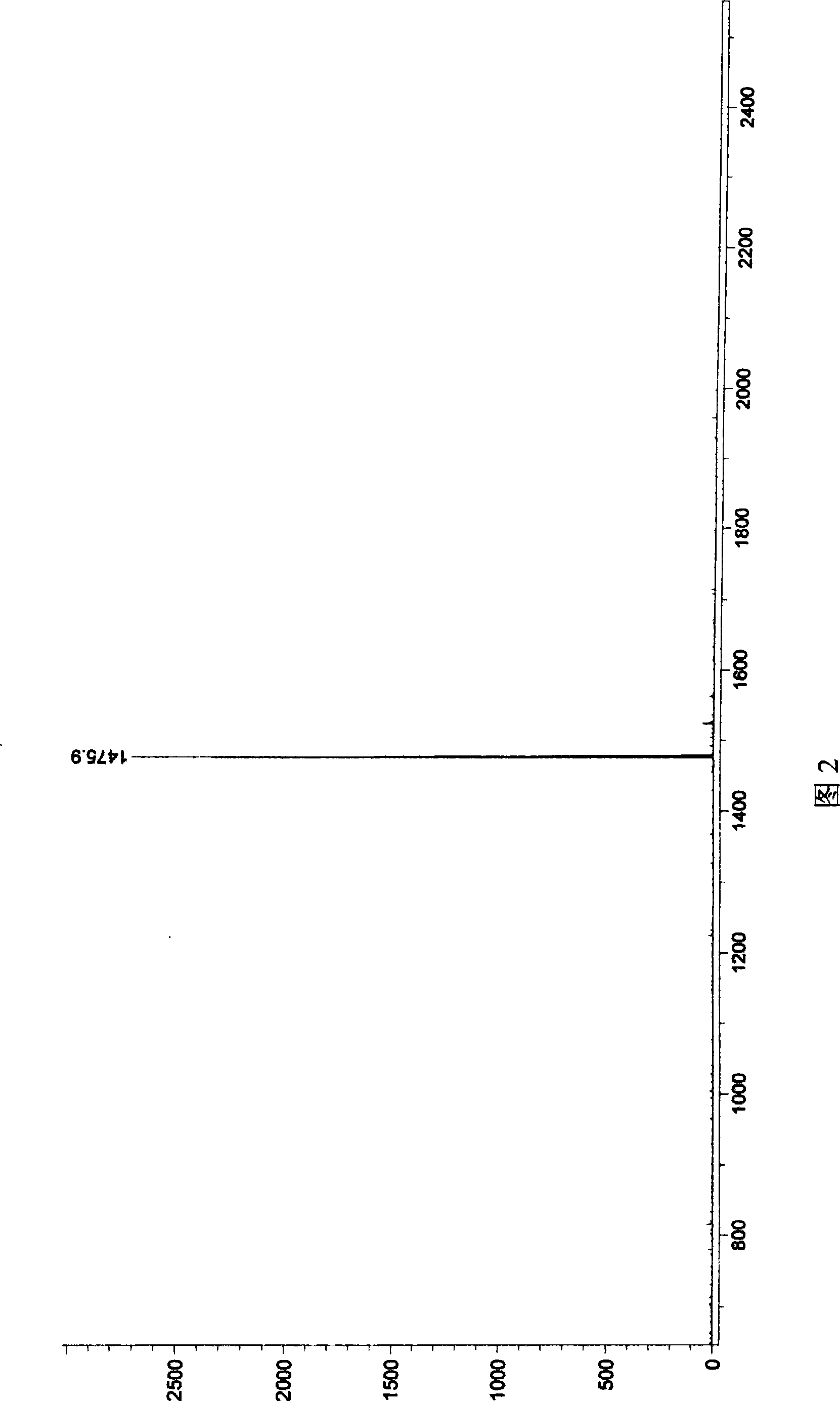
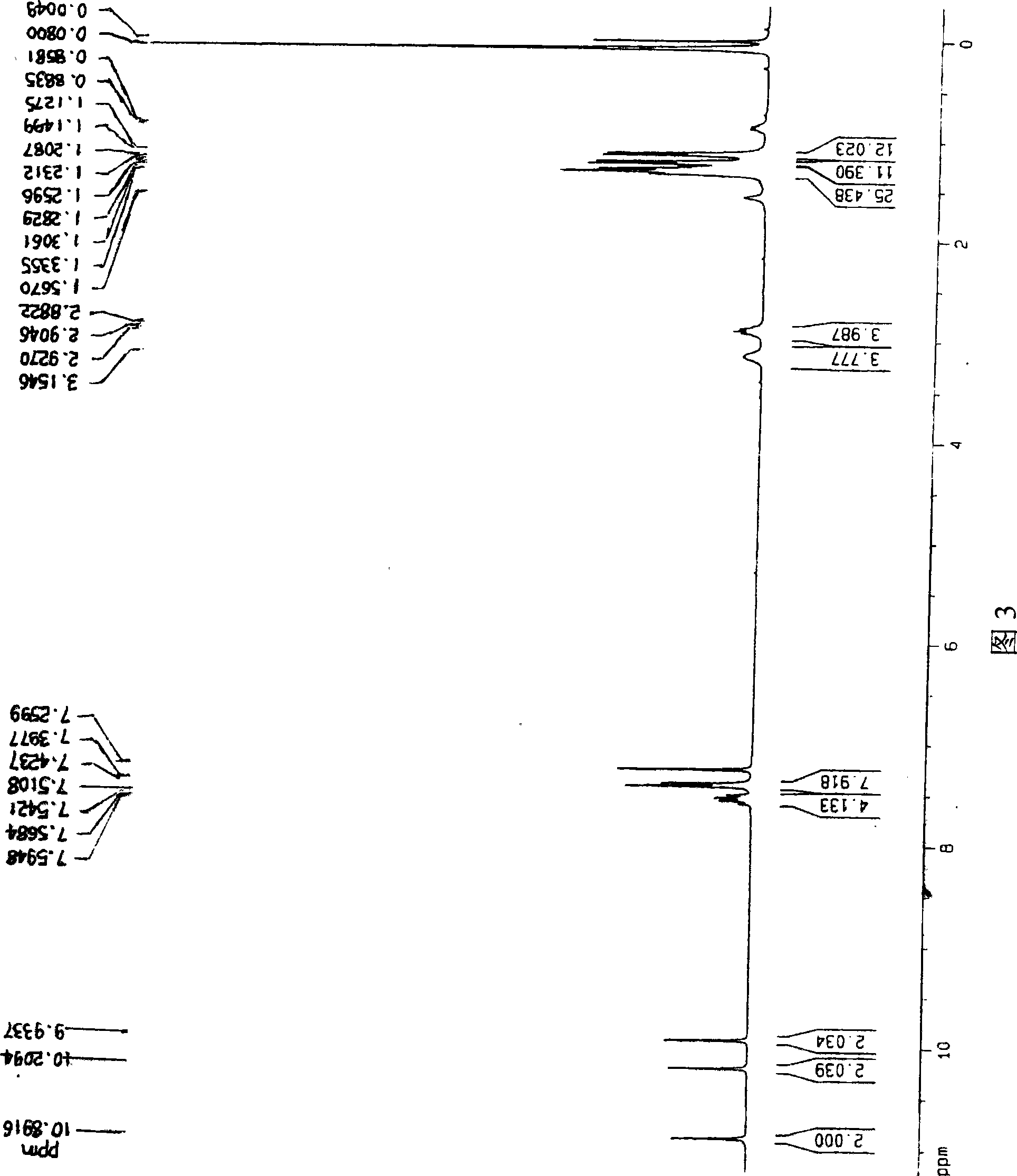
[0057] N,N',N",N"'-tetrakis(2,6-diisopropylphenyl)-thiabiperylene-3,4,6,7:12,13,15,16-octacarboxylic The mass spectrogram of acid tetraimide is shown in Figure 1; , 6,7: The mass spectrum of 12,13,15,16-octacarboxylic acid tetraimide is shown in Figure 2; 1 The H-NMR figure is shown in Figure 3; 13 The C-NMR chart is shown in FIG. 4 . Example 2 (N, N', N", N"'-tetrakis(2,6-diisopropylphenyl)-selenobiperylene-3,4,6,7:12,13,15, 16-Octacarboxylic tetraimide and N,N',N",N"'-tetrakis(2,6-diisopropylphenyl)-diselenobiperylene-3,4,6,7 : 12,13,15,16-octacarboxylic acid tetraimide)
[0058]
[0059] Concrete synthetic steps are:
[0060] 1. Add 1mmol of N,N'-bis(2,6-diisopropylphenyl)-1,6,7,12-tetrachloroperylene-3,4:9,10-tetracarboxylic diimide , 12mmol sodium selenide, 6mmol cuprous iodide, 7mmol L-proline and 14mmol anhydrous potassium carbonate are mixed together;
[0061] 2. Add 10ml dimethyl sulfoxide to the mixture prepared in step 1;
[0062]3. Under nitrogen protect...
Embodiment 3
[0064] Example 3 (N, N', N", N"'-tetrakis(2,6-diisopropylphenyl)-thiabiperylene-3,4,6,7:12,13,15, 16-Octacarboxylic tetraimide and N,N',N",N"'-tetrakis(2,6-diisopropylphenyl)-dithiabiperylene-3,4,6,7 : 12,13,15,16-octacarboxylic acid tetraimide)
[0065]
[0066] Concrete synthetic steps are:
[0067] 1. Add 1 mmol N, N'-bis(2,6-diisopropylphenyl)-1,6,7,12-tetrabromoperylene-3,4:9,10-tetracarboxylic diimide , 14mmol sodium sulfide, 7mmol cuprous chloride, 8mmol L-proline and 11mmol anhydrous potassium carbonate are mixed together;
[0068] 2. Add 12ml dimethyl sulfoxide to the mixture prepared in step 1;
[0069] 3. Under nitrogen protection, heat the mixed solution prepared in step 2 to 80°C and react for 10 hours; after the reaction, the product is dissolved in ethyl acetate, washed with aqueous sodium chloride, dried over anhydrous sodium sulfate, filtered, The solvent was evaporated to dryness below 60°C, and the product N, N', N", N"'-tetrakis(2,6-diisopropylphenyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com