Inspection method of streptococcus in cosmetics
An inspection method and technology of cosmetics, which is applied in the field of cosmetic microbial inspection and bacteria inspection in cosmetics, and can solve problems such as dermatitis, folliculitis, boils, and no detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
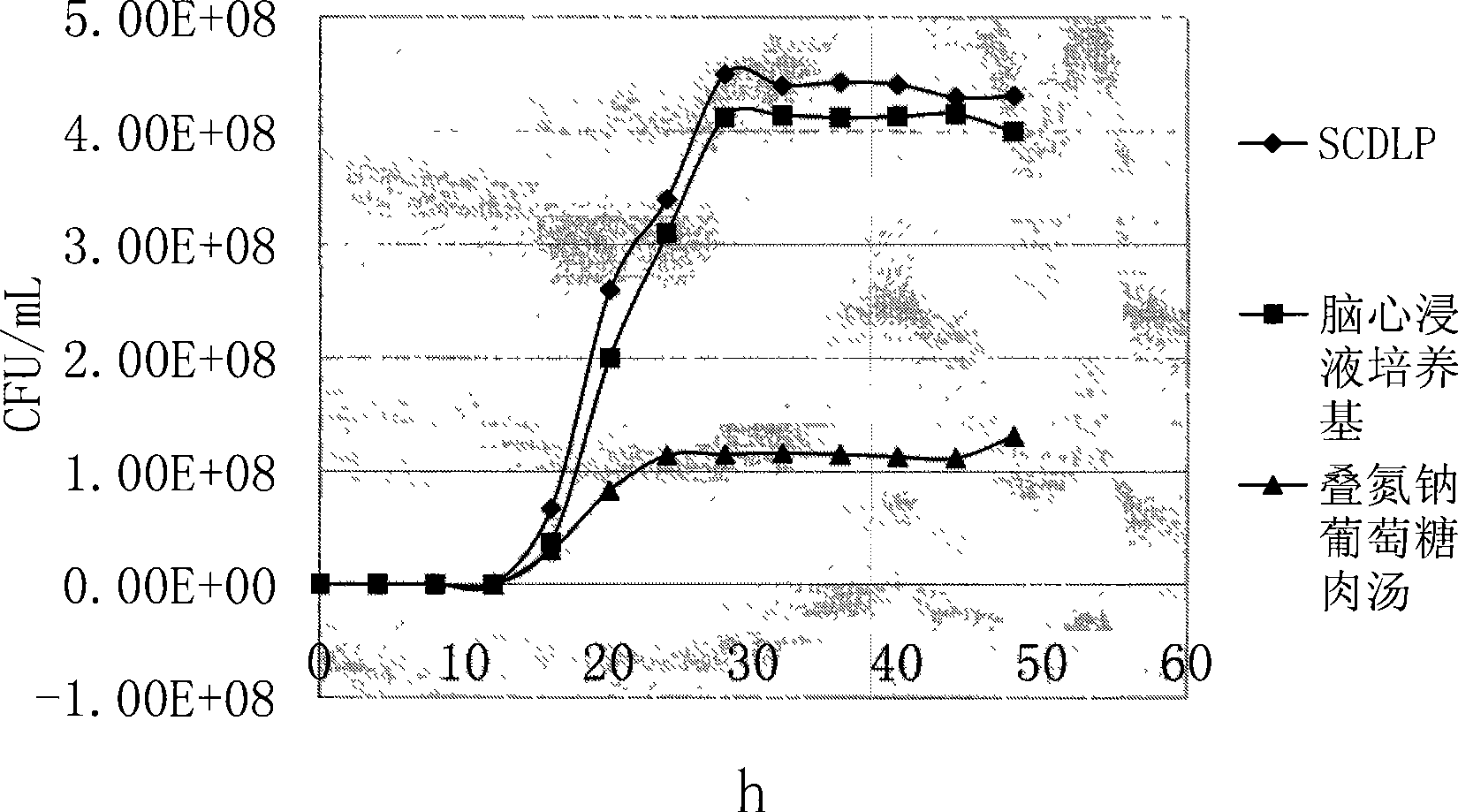
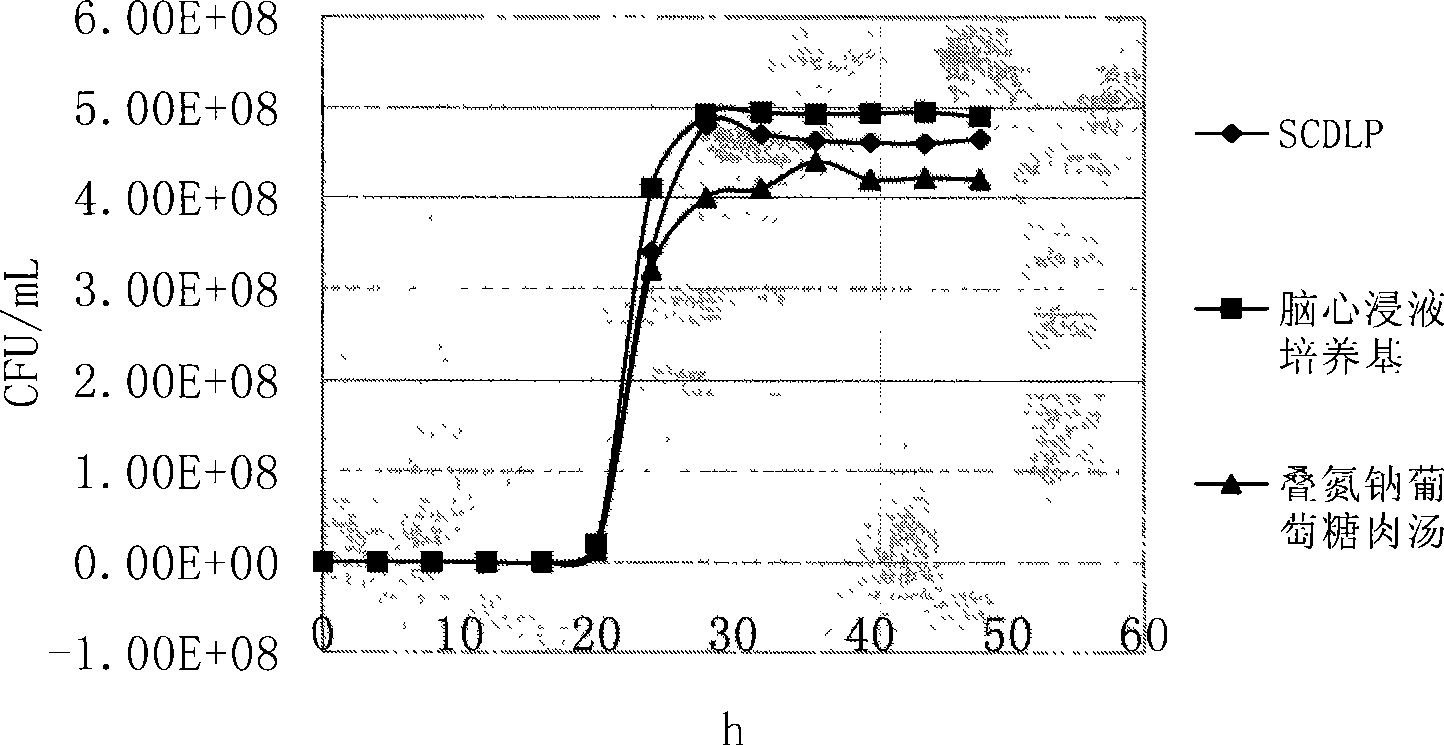
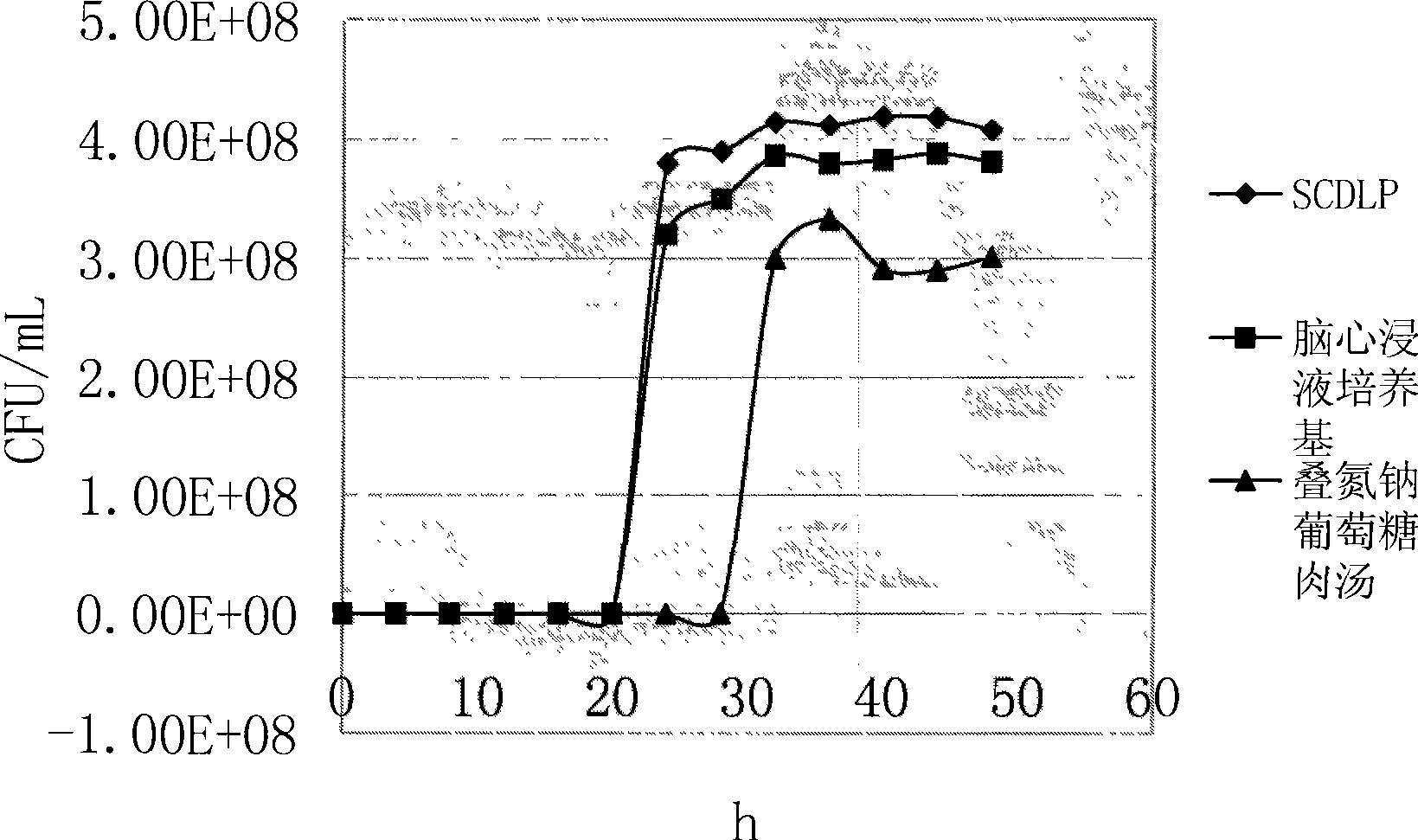
[0037] In order to determine whether SCDLP, brain heart infusion medium and sodium azide glucose broth can be used as pre-enrichment solutions for Streptococcus, five standard strains were added to the above three pre-enrichment media, cultured at 37°C, and each Draw 1 mL of culture solution from each enrichment solution every 4 hours, and after ten-fold gradient dilution with sterile normal saline, select the appropriate dilution and apply it to the brain heart infusion agar, and count the colonies. The experiment was carried out continuously for 48 hours. Compare their growth curves to determine the optimal enrichment medium.
[0038] Streptococcus constellation CMCC(B)32116, Streptococcus pyogenes CMCC(B)32002, Streptococcus faecalis CMCC(B)32223, Streptococcus alpha-hemolyticus CMCC(B)32205 and Streptococcus lactis were selected as the standard bacteria, respectively. It was made into a 0.5 McFarland concentration of bacteria, diluted ten times with sterile normal saline, ...
Embodiment 2
[0042] To determine whether blood agar, brain heart infusion agar and KF agar are suitable as isolation media for streptococci. The 5 standard strains with the same amount and appropriate concentration were respectively inserted into blood agar, brain heart infusion agar and KF agar, counted after culturing at 37°C for 24 hours, and observed the growth characteristics and number of growth in the three agar media. , to determine the optimal isolation medium for streptococci.
[0043] Streptococcus constellation CMCC(B)32116, Streptococcus pyogenes CMCC(B)32002, Streptococcus faecalis CMCC(B)32223, Streptococcus alpha-hemolyticus CMCC(B)32205 and Streptococcus lactis were selected as the standard bacteria, respectively. It was made into a bacterial solution of 0.5 McFarland concentration, diluted ten times with sterile physiological saline, and 1 mL was coated on blood agar, brain heart infusion agar and KF agar, cultivated at 37 °C for 24 hours and counted, and counted each sta...
Embodiment 3
[0047] Example 3: Isolation of Streptococcus by Artificial Pollution of Cosmetics
[0048] Streptococcus constellation CMCC(B)32116, Streptococcus pyogenes CMCC(B)32002, Streptococcus faecalis CMCC(B)32223, Streptococcus alpha-hemolyticus CMCC(B)32205 and Streptococcus lactis were selected as the standard bacteria, respectively. It is made to be adjusted to 0.5 McFarland concentration, and mixed into 6 kinds of cosmetics (skin rejuvenation lotion (liquid), skin softening lotion (liquid), facial cleanser (ointment), moisturizing lotion (emulsion semi-emulsion) according to the amount of 1%. solid), nutritional cream (oily), perfume (aerosol and organic solvent)), mix well. The fully mixed liquid, semi-solid and other samples were prepared with reference to GB7918.1-87 (General Principles of Microbiological Standard Test Methods for Cosmetics). Take 10 mL of the 1:10 diluted sample and inoculate it into 90 mL of SCDLP liquid medium, set it in a 37 °C incubator, and cultivate fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com