High function type eletrolysis solution used for lithium ionic cell
A lithium-ion battery and electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve the problems of poor low-temperature performance, insufficient stability of the SEI film on the surface of the negative electrode, low solubility and conductivity, etc., to improve conductivity, Excellent electrochemical stability and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a glove box filled with argon gas, weigh 38% GBL, 38% EA, 19% EC and 5% VC by weight. After mixing well, slowly add 0.7mol / L electrolyte salt. LiBOB, stir until the lithium salt is completely dissolved, and then the high-functional electrolyte of the present invention can be obtained, and the ratio is 5wt.% VC+0.7mol / LLiBOB-GBL:EA:EC (1:1:0.5, wt).
[0022] At room temperature (20°C), the conductivity of the electrolyte in Test Example 1 was 9.05 mS·cm -1 .
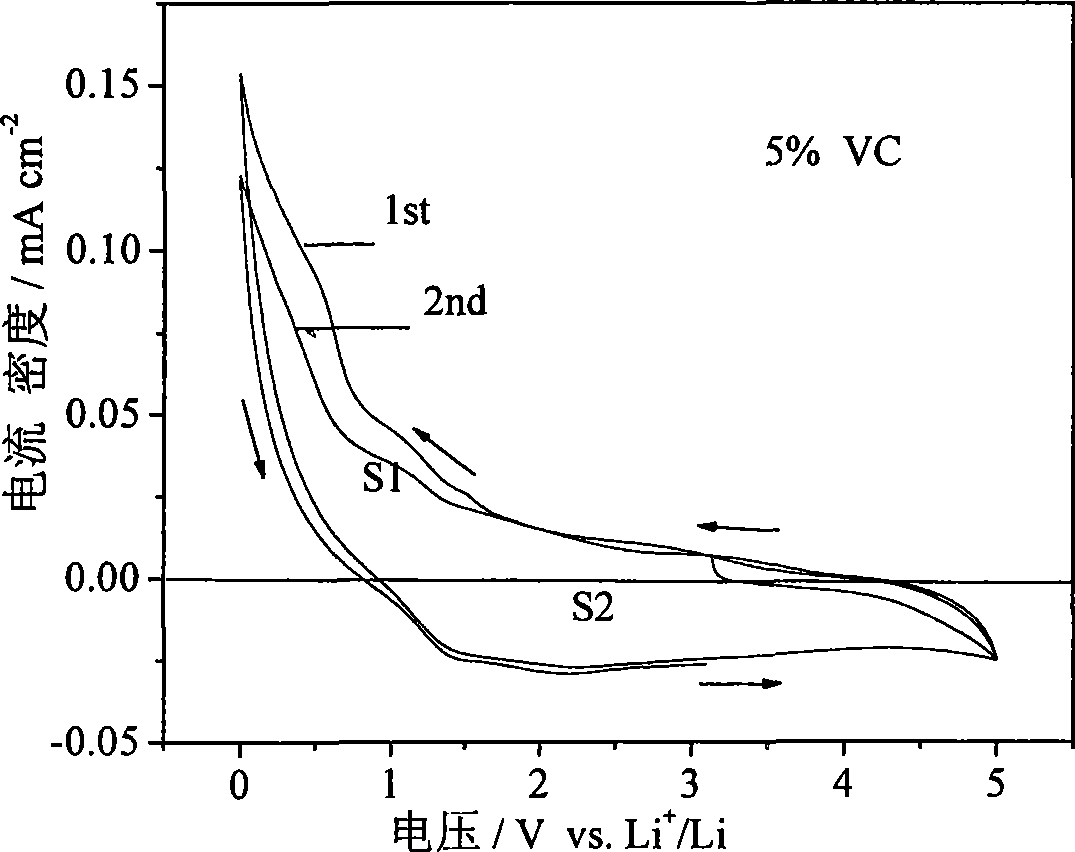
[0023] The electrochemical stability test result of embodiment 1 electrolyte is as follows figure 1 shown. figure 1 The medium current density is relatively small, and the electrolyte can be considered to be relatively stable. When the current density reaches 0.1mA / cm -2 , it can be considered that the electrolyte begins to decompose. The electrochemical window of the high-functional electrolyte is 0.4-5V. After the first scan, figure 1 S1 and S2 are roughly equal, and the reaction is reversible.
[0024...
Embodiment 2
[0027] In a glove box filled with argon gas, weigh 38% GBL, 38% EA, 19% EC and 5% ES respectively by weight. After mixing well, slowly add 0.7mol / L electrolyte salt. LiBOB, stir until the lithium salt is completely dissolved, and then the high-performance electrolyte of the present invention can be obtained, and the ratio is 5wt.% ES+0.7mol / LLiBOB-GBL:EA:EC (1:1:0.5, wt).
[0028] At room temperature (20°C), the conductivity of the electrolyte in Example 2 is 8.96 mS·cm -1 .
[0029] The electrochemical stability test result of embodiment 2 electrolyte is as follows Figure 4 shown. Figure 4 The current density in is relatively small, and it can be considered that the electrolyte is relatively stable. When the current density reaches 0.1mA / cm -2 , it can be considered that the electrolyte begins to decompose. The electrochemical window of the high-functional electrolyte is 0.5-5V. After the first scan, Figure 5 S1 and S2 are roughly equal, and the reaction is reversib...
Embodiment 3
[0033]In an argon-filled glove box, weigh 48% GBL, 38% EA, 10% EC, 2% ES, and 2% VC by weight. After mixing well, slowly add 1.0 mol / L electrolyte salt LiBOB is stirred until the lithium salt is completely dissolved, and then the high-performance electrolyte of the present invention can be obtained.
[0034] At room temperature (20°C), the electrical conductivity of the quinary electrolyte of Example 3 obtained by testing was 8.71 mS·cm -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com