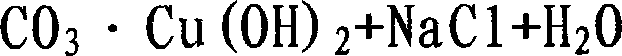Process for producing basic copper carbonate
A copper carbonate and basic technology, applied in chemical instruments and methods, copper compounds, inorganic chemistry, etc., can solve problems such as the inability to meet market needs and the inability of production technology to achieve purity, achieving low cost, low waste water, and purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
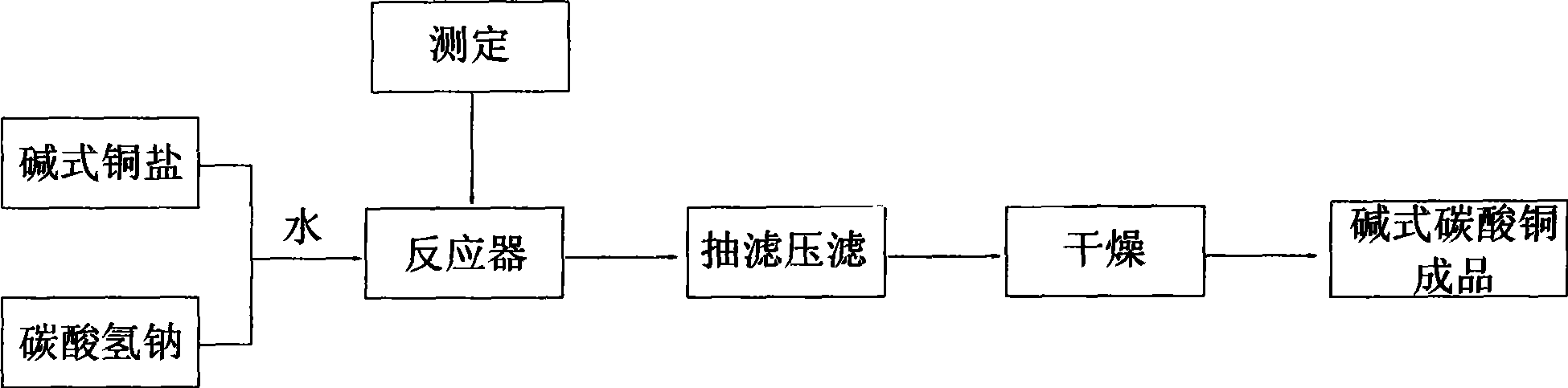
[0013] Such as figure 1 Shown, the preparation method of basic copper carbonate of the present invention comprises the following processing steps:
[0014] Stir the solid state of basic copper salt (including basic copper nitrate or basic copper sulfate or basic copper chloride or copper hydroxide) or copper hydroxide with water in a reactor to form a slurry; add the solid to the reactor Sodium bicarbonate is used for solid-state reaction, and the temperature is controlled between 20°C and 140°C;
[0015] Among them, the chemical reaction formula is:
[0016]
[0017]
[0018]
[0019]
[0020]
[0021]
[0022] After the conversion is determined, put down the basic copper carbonate slurry, press filter or suction filter and wash up to the standard, and then dry to obtain the basic copper carbonate finished product.
[0023] In the above-mentioned reactions, the raw material basic copper chloride is very rich. Since my country is a big producer of electroni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com