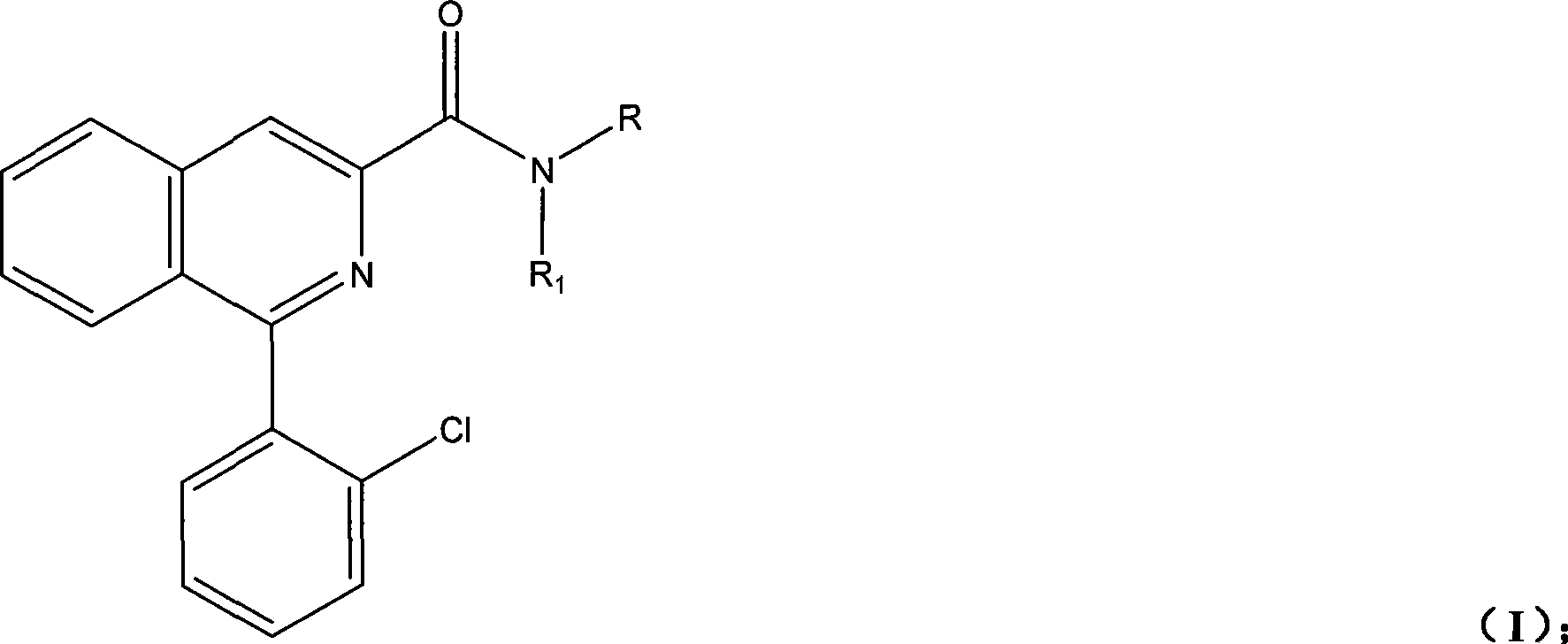
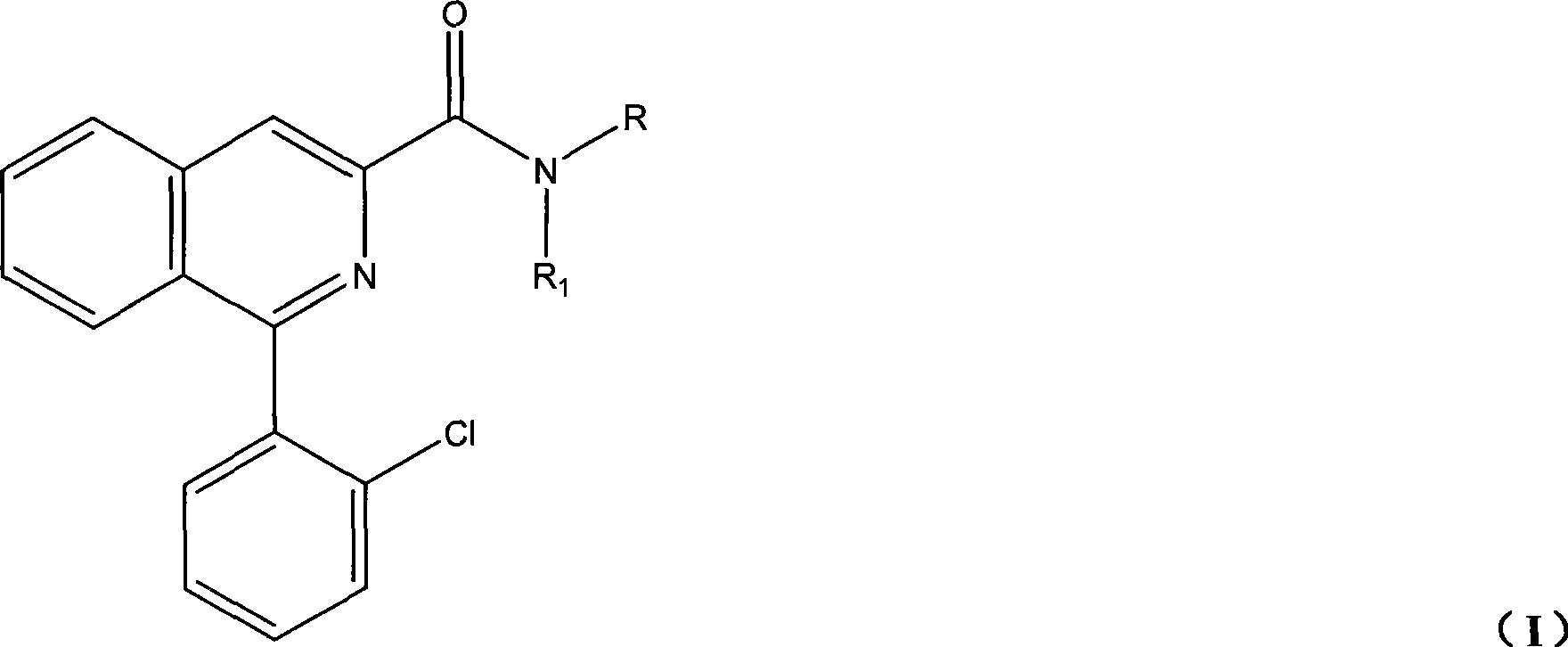
Synthesis of PET imaging agent prosome isoquinoline methanamide derivant
A technology of PET imaging agent and isoquinoline formamide, which is applied in the new synthesis of 1-isoquinoline-3-carboxamide compounds and the synthesis field of isoquinoline formamide derivatives of PET imaging agent marking precursors , can solve the problems of low yield of PET imaging agent labeling precursor, cumbersome steps, complicated operation, etc., and achieve the effect of simplifying industrial production equipment and production process, simple process and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Preparation of 1-(2-chlorophenyl)-N-isobutylisoquinoline-3-carboxamide
[0068] Preparation of 2-chlorohippuric acid:
[0069] Place 250ml of 3% sodium hydroxide solution in an ice bath for cooling, add 15g of glycine and stir to dissolve, then slowly drop a mixed solution of 25ml of o-chlorobenzoyl chloride and 100ml of toluene. After the dropwise addition was completed, the reaction was continued at room temperature for 3 hours. After the reaction is complete, adjust the pH to 2-3 with dilute hydrochloric acid. Stand still, filter with suction, and dry to obtain white crystals. The white crystals were dried and weighed. 34.1 g of white crystals were obtained with a yield of 79.8%.
[0070] IRv(KBr) / cm -1 : 3286, 1724, 1628;
[0071] 1 H-NMR: 4.18 (d, 2H, J = 5.8 Hz), 7.39-7.85 (m, 5H).
[0072] Preparation of 4-benzylidene-2-(2-chlorophenyl)oxazol-5-one:
[0073] Add 5.340g of 2-chlorohippuric acid, 2.660g of benzaldehyde, 3.190g of sodium acetate and 75ml of...
Embodiment 2
[0089] Preparation of 1-(2-chlorophenyl)-N-tert-butylisoquinoline-3-carboxamide
[0090] Preparation of 2-chlorohippuric acid:
[0091] Place 250ml of 15% sodium hydroxide solution in an ice bath for cooling, add 15g of glycine and stir to dissolve, then slowly drop a mixed solution of 15ml of o-chlorobenzoyl chloride and 100ml of toluene. After the dropwise addition was completed, the reaction was continued at room temperature for 2 hours. After the reaction is complete, adjust the pH to 3 with dilute hydrochloric acid. Stand still, filter with suction, and dry to obtain white crystals. The white crystals were dried and weighed. 35.2 g of white crystals were obtained with a yield of 81.3%.
[0092] IRv(KBr) / cm -1 : 3286, 1724, 1628;
[0093] 1 H-NMR: 4.18 (d, 2H, J = 5.8 Hz), 7.39-7.85 (m, 5H).
[0094] Preparation of 4-benzylidene-2-(2-chlorophenyl)oxazol-5-one:
[0095] Add 5.340g of 2-chlorohippuric acid, 3.320g of benzaldehyde, 3.190g of sodium acetate and 75ml of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com