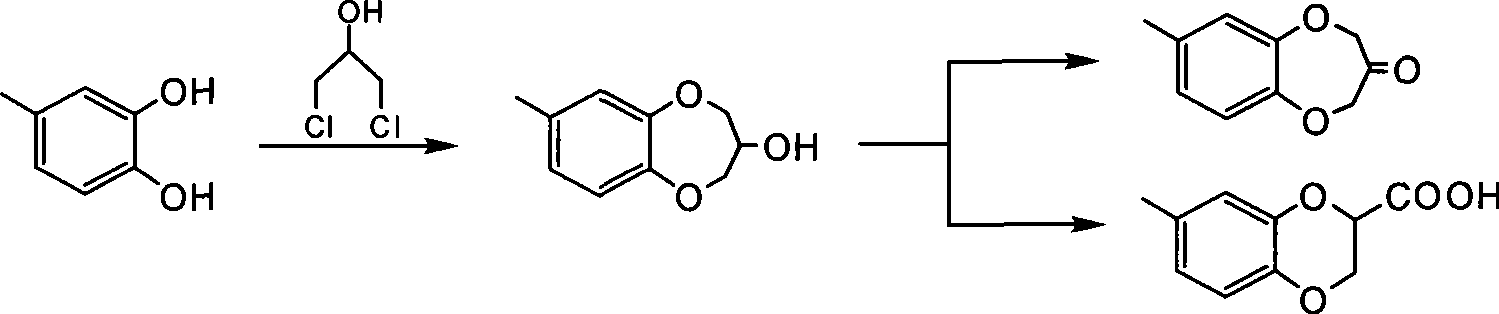
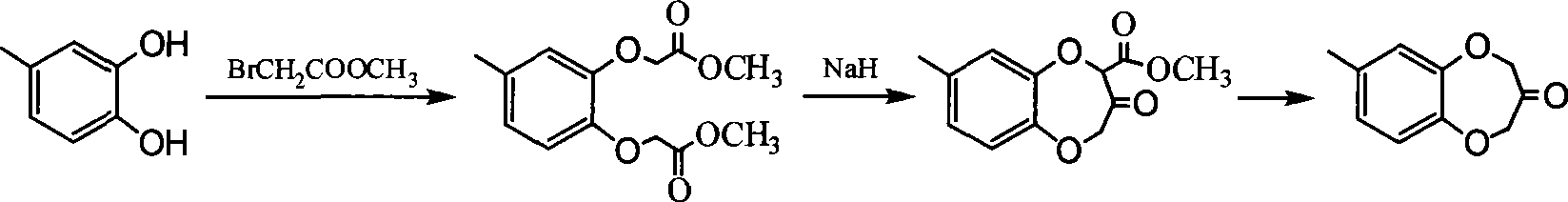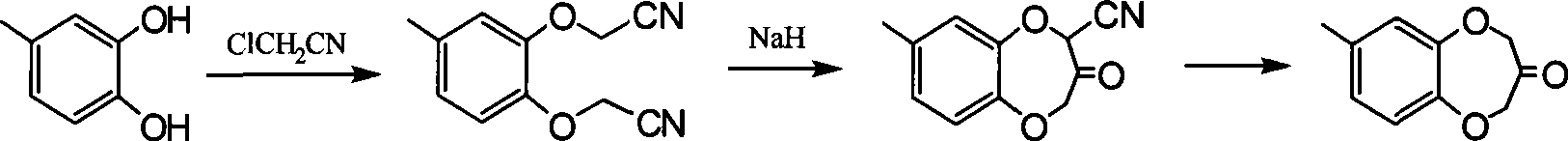Synthesis of watermelon ketone
A synthesis method and watermelon ketone technology are applied in the synthesis field of fragrance compounds, can solve the problems of harsh reaction conditions, low reaction yield, lack of general application prospects, etc., and achieve the effects of convenient operation, mild reaction conditions and high product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 2000ml methanol to a 5000ml four-necked round bottom flask equipped with a thermometer, a reflux condenser and a stirrer, protect it under nitrogen, add 400g triethylamine, 40g potassium iodide, 300g4-methylcatechol, 10gPEG-400 in turn, and heat To 65°C, slowly add a mixed solution of 300g of 3-dichloroacetone and 350ml of methanol dropwise, and continue to insulate and reflux for 5 hours after the drop; during the reaction process, take samples for GC analysis, cool, and filter with suction to obtain the crude product, and the filtrate removes methanol at 80°C Add 600ml of ethyl acetate to the crude product, wash with saturated brine until neutral, and recover ethyl acetate; Distill under reduced pressure, collect 270 grams of watermelon ketone fraction, and use 500 grams of 1:2 methanol and n-hexane mixed solvent for secondary crystallization of the fraction , to obtain 195 grams of white crystalline watermelon ketone, with a content of 98.9%, and a yield of 45.3%....
Embodiment 2
[0020] Add 2200ml of methanol into a 5000ml four-necked round bottom flask equipped with a thermometer, reflux condenser, and stirrer, protect it with nitrogen, add 500g of pyridine, 50g of potassium iodide, 350g of 4-methylcatechol, and 17.5g of PEG-400 in sequence, and heat to 68°C, slowly add dropwise a mixed solution of 350g of 3-dichloroacetone and 450ml of methanol, and continue to insulate and reflux for 5.5 hours after the drop; during the reaction process, take samples for GC analysis, cool, and suction filter to obtain the crude product, and the filtrate is removed below 80°C Remove methanol; add 500ml ethyl acetate to the crude product, wash with saturated brine until neutral, and recover ethyl acetate; distill under reduced pressure, collect 290 grams of watermelon ketone fraction, and use 650 grams of 1:2 methanol:n-hexane mixed solvent for the fraction Secondary crystallization gave 216 grams of white crystalline watermelon ketone product with a content of 98.9% a...
Embodiment 3
[0022] Add 2000ml methanol to a 5000ml four-necked round-bottomed flask equipped with a thermometer, a reflux condenser, and an agitator. Under nitrogen protection, add 425g triethylamine, 45g potassium iodide, 300g4-methylcatechol, and 12.5gPEG-400 in sequence. Heat to 70°C, slowly add a mixed solution of 300g of 3-dichloroacetone and 350ml of methanol dropwise, and continue to insulate and reflux for 6 hours after the drop; during the reaction, take samples for GC analysis, cool, and filter with suction to obtain the crude product, and the filtrate is removed at 80°C Methanol; add 600ml of ethyl acetate to the crude product, wash with saturated saline until neutral, and recover ethyl acetate; distill under reduced pressure, collect 270 grams of watermelon ketone fraction, and use 500 grams of 1:2 methanol and n-hexane mixed solvent for the second fraction Crystallized to obtain 208 grams of white crystalline watermelon ketone, with a content of 99.1%, and a yield of 46.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com