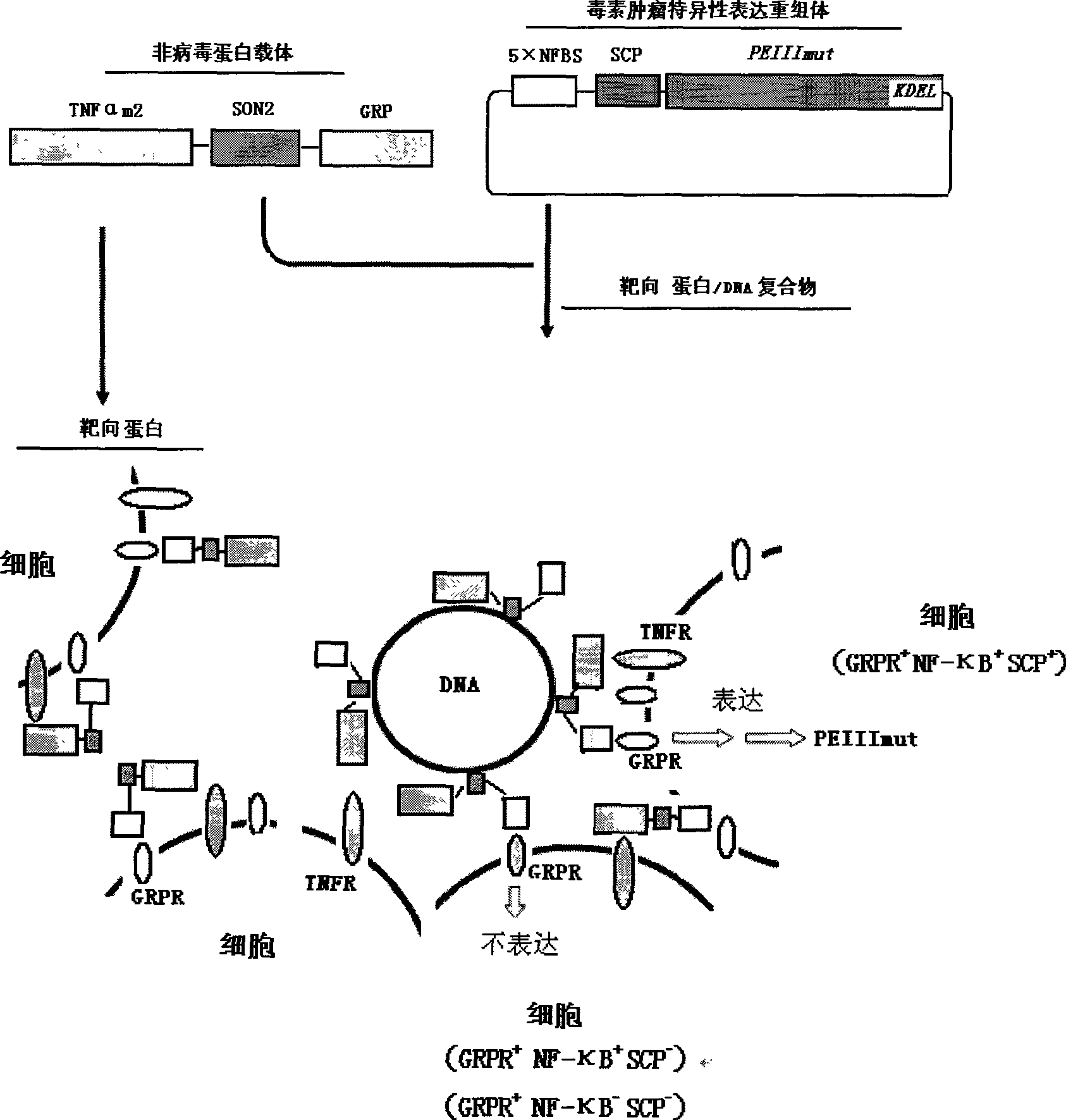
Type T non--viral vector and composite medicament containing the same
A non-viral carrier and drug technology, applied in the direction of using vectors to introduce foreign genetic material, drug combinations, anti-tumor drugs, etc., can solve problems such as high cost, antibody neutralization and inactivation, and allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Example 1 Construction of recombinant plasmid 5×NFBS-SCP-Luc (ie 5×κ B sites-survivin core promoter-luciferase)
[0163] 1.1 Cloning of SCP (survivin core promoter) sequence
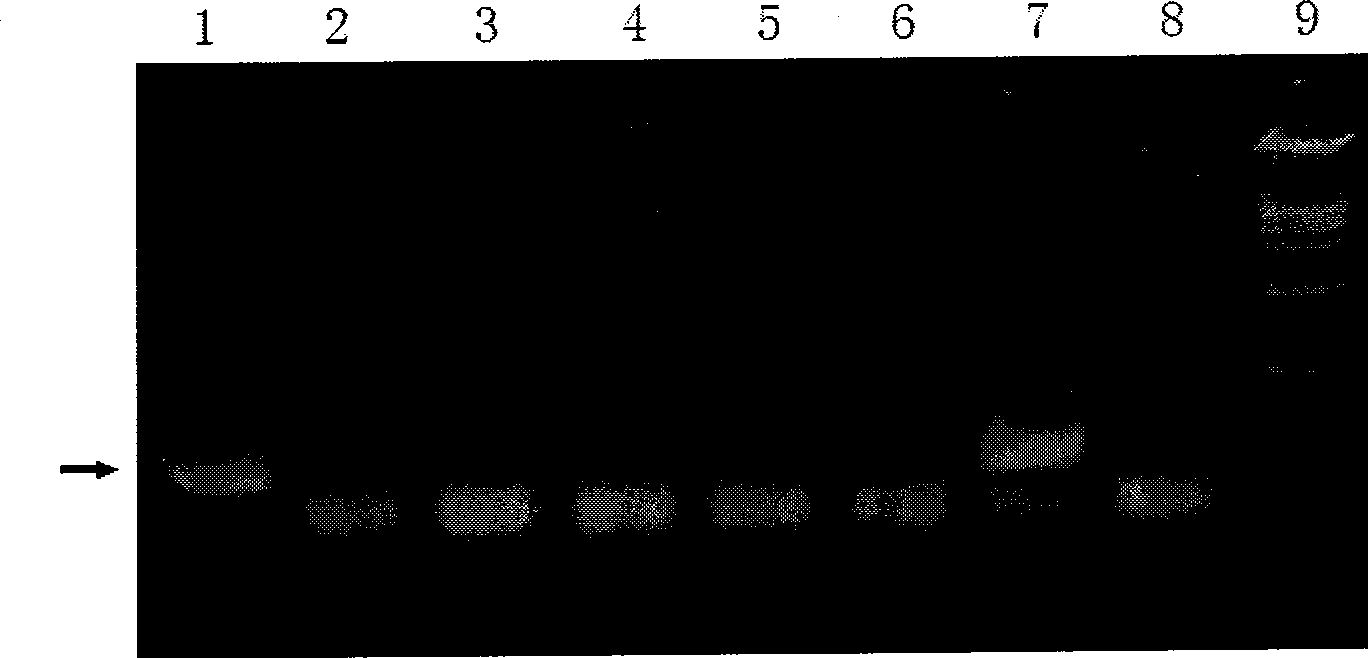
[0164] It has been confirmed that the gene expression guided by the SCP with a length of 269 bp located at positions 2543-2811 of the DNA sequence (14796 bp) of the human survivin gene has tumor specificity. To evaluate it using the luciferase reporter gene expression system, the SCP will be inserted into the vector through the Nhe I and Sma I sites in the vector MCS. Firstly, it was inserted into pMD18-T simple vector [purchased from Takara Bioengineering (Dalian) Co., Ltd. (TAKARA)]. Primers were designed according to the DNA sequence of the human survivin gene provided by GenBank, and the PCR reaction was carried out with human genomic DNA as a template to obtain a 287bp target fragment-Nhe I—SCP—Sma I—(see figure 2 ). This fragment was ligated with the linear pMD18-T simple vector. The li...
Embodiment 2
[0170] Example 2 Construction of other various luciferase reporter gene expression recombinants
[0171] 2.1 Construction of recombinant plasmid SCP-Luc-Esv40 (survivin core promoter-luciferase-SV40 enhancer)
[0172] The 277bp fragment Nhe I—SCP—Sma I from Example 1.3 was inserted into the plasmid pGL3-Enhancer through the sites Nhe I and Sma I. It has been confirmed that the correct recombinant plasmid SCP-Luc-Esv40 has been obtained.
[0173] 2.2 Construction of recombinant plasmid 5×NFBS-Luc (ie κ B site-luciferase)
[0174] The 93bp fragment Kpn I-5×NFBS-Sac I from Example 1.4 was inserted into the plasmid pGL3-Basic via the sites Kpn I and Sac I. It has been confirmed that the correct recombinant plasmid 5×NFBS-Luc has been obtained.
[0175] 2.3 Construction of recombinant plasmid 5×NFBS-SCP-Luc-Esv40 (ie κ B sites-survivin core promoter-luciferase-SV40 enhancer)
[0176] The 93bp fragment Kpn I-5×NFBS-Sac I from Example 1.4 was inserted into the recombinant plasmid...
Embodiment 3
[0177] Example 3 The liposome-mediated transient transfection of the luciferase reporter gene expression recombinant was used to evaluate the tumor specificity of the gene expression guided by the regulatory sequence.
[0178] 3.1 A large amount of the above-mentioned luciferase expression vectors were extracted.
[0179] Includes: (1) Psv40-Luc-Esv40 (2) SCP-Luc (3) 5×NFBS-SCP-Luc
[0180] (4)SCP-Luc-Esv40 (5)5×NFBS-SCP-Luc-Esv40 (6)5×NFBS-Luc
[0181] 3.2 Detection of gene expression guided by various forms of regulatory sequences
[0182] Mediated by liposomes, the above-mentioned luciferase expression vectors were respectively applied to three tumor cell lines (human breast cancer cell lines MCF-7 and MDA-MB-231, and human cervical cancer cell line HeLa; all three were NF-κ B + SCP + ); and a non-tumor cell line (human embryonic skin fibroblast cell line CCC-ESF-1, for NF-κB + SCP - ) were transiently transfected; and the harvested cells were detected and analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com