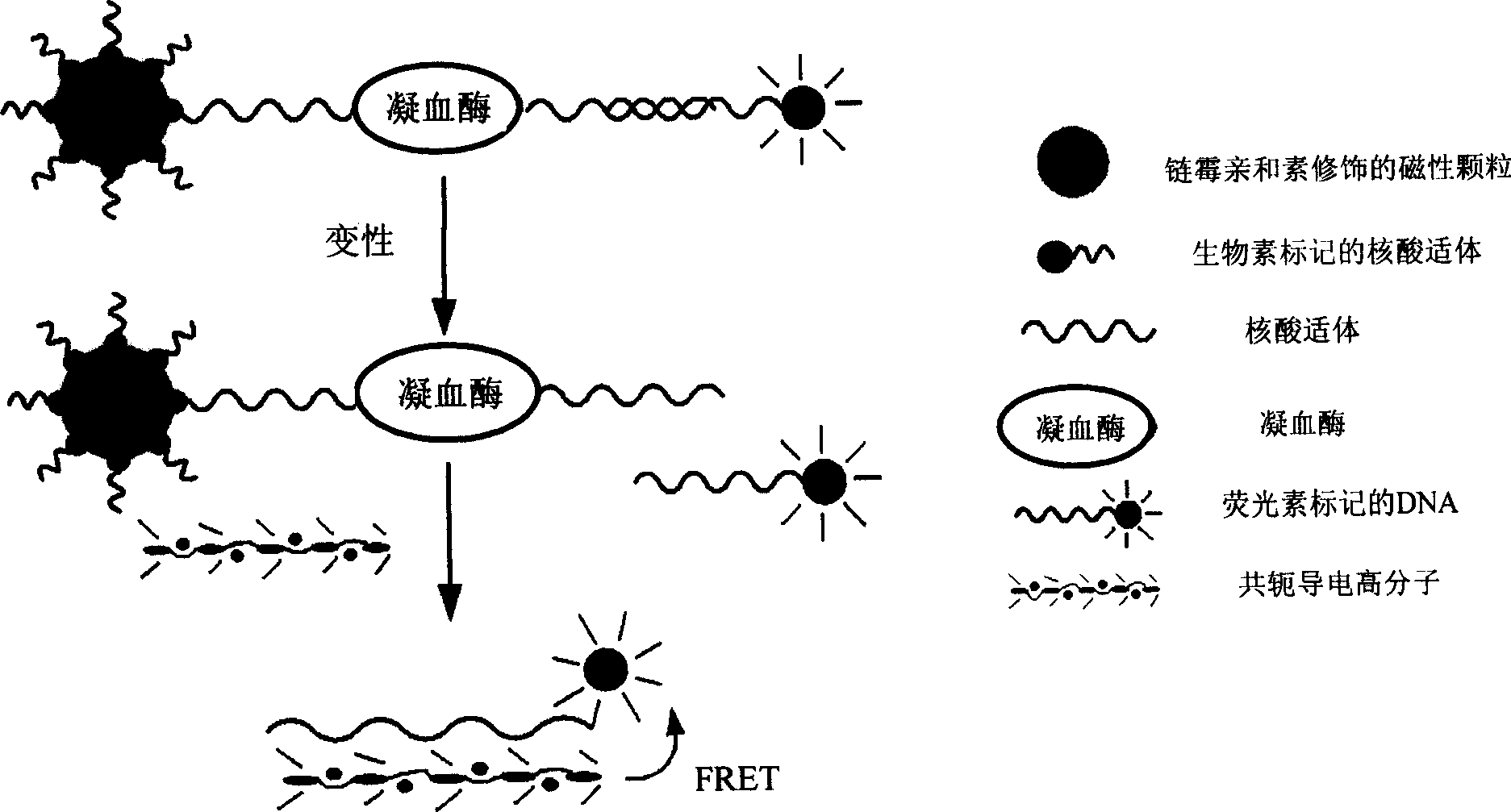
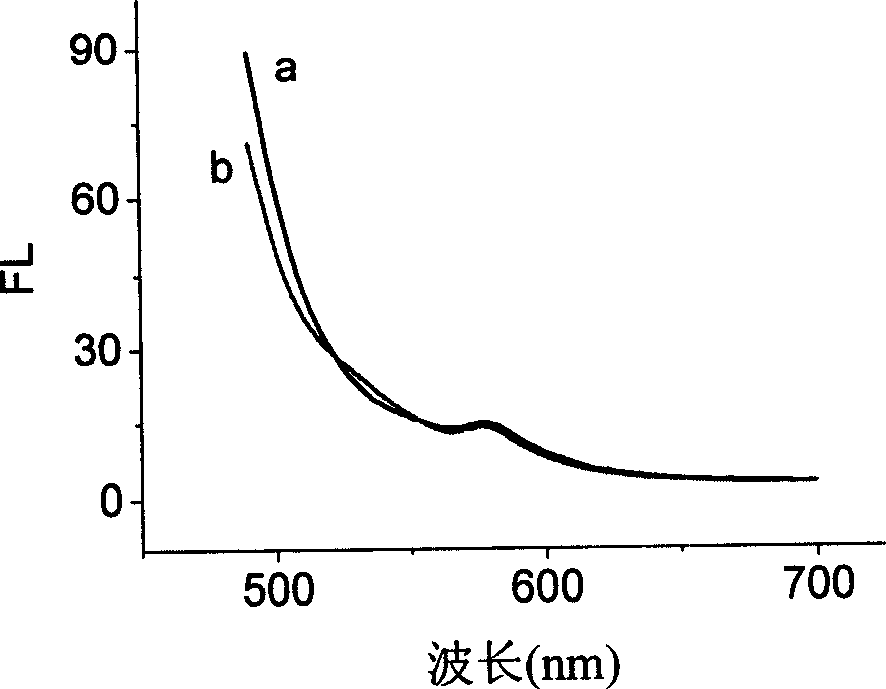
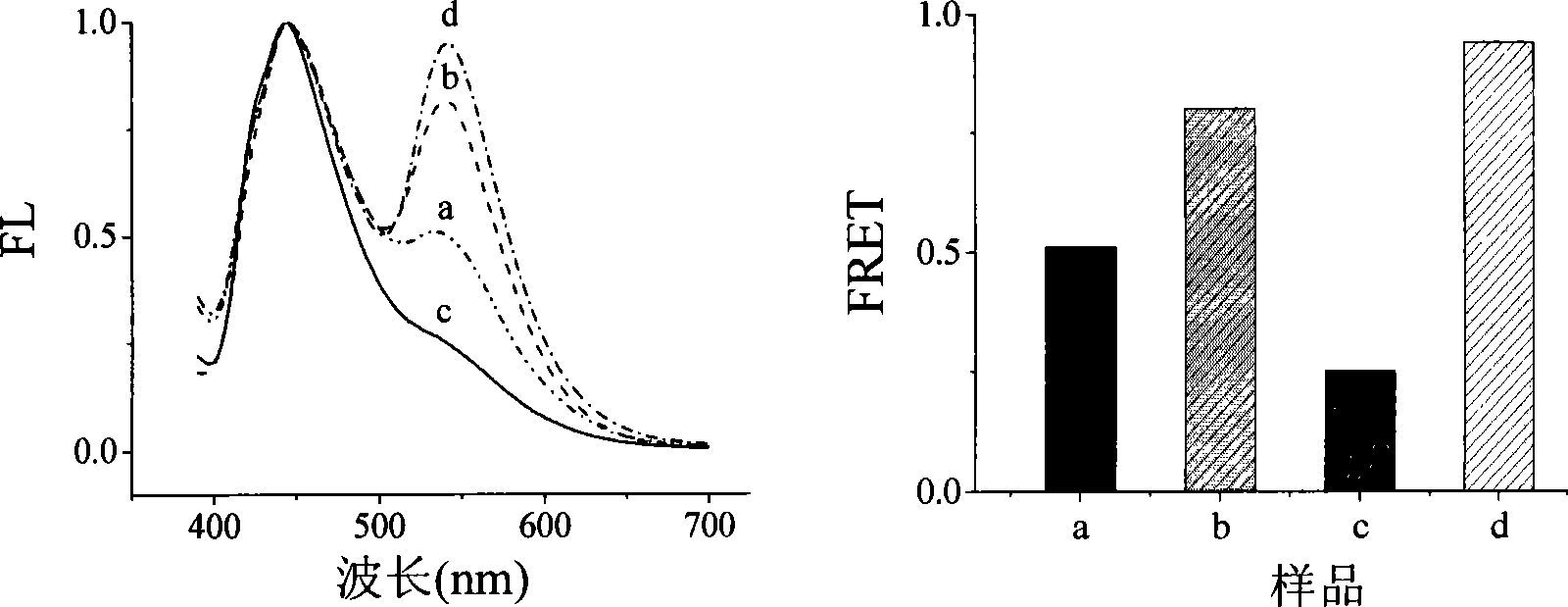
Detection method for target substance based on aptamer and its solid phase biological inductor
A nucleic acid aptamer and biosensor technology, applied in the field of biosensors, can solve problems such as short detection time, difficult to distinguish signals, and limited sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of Magnetic Particle-Capture Probe Complex
[0050] According to the product instructions, MMPs were washed 3 times with 0.5×SSC buffer before use, and then 5 μl of biotin-labeled capture probe 2 with a concentration of 25 μM was added to 100 μl of TTL buffer containing 10 μg of MMPs, at room temperature Mix gently for 10 minutes. The surface density of capture probes is about 4-6×10 11 chain / cm 2 . Then, the complex of MMPs and capture probe (MMPs-capture probe, denoted as MMPs-cp1) was washed twice with TTA buffer, suspended in the binding solution at a suspension concentration of 1.25 μM, and refrigerated at 4°C for use.
Embodiment 2
[0051] Embodiment 2 bridge molecule and signal probe hybridization
[0052] 2.17 μl of bridge molecules with a concentration of 46 μM and 1.62 μl of signal probes with a concentration of 62 μM were added to 50 μl of hybridization buffer (the composition of the hybridization buffer was 750 mM NaCl, 75 mM sodium citrate, pH 7.4), and hybridized at room temperature for 20 minutes. A double-stranded signal probe was obtained.
Embodiment 3
[0053] Example 3 Blocking of vacant sites in the magnetic particle-capture probe complex
[0054] Take 0.1 ml of the suspension of the magnetic particle-capture probe complex (MMPs-cp1) prepared above and refrigerated at 4°C, wash with TTA buffer three times, magnetically separate and suck the supernatant, add 0.1 The bovine serum albumin (BSA) solution with a ml concentration of 2 wt % was blocked at room temperature for 30 minutes. Then wash twice with TTA buffer, suspend in the binding solution, the suspension concentration of the magnetic particle-capture probe complex in the suspension is the same as before blocking, and refrigerate at 4°C for future use (marked as MMPs-cp2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com