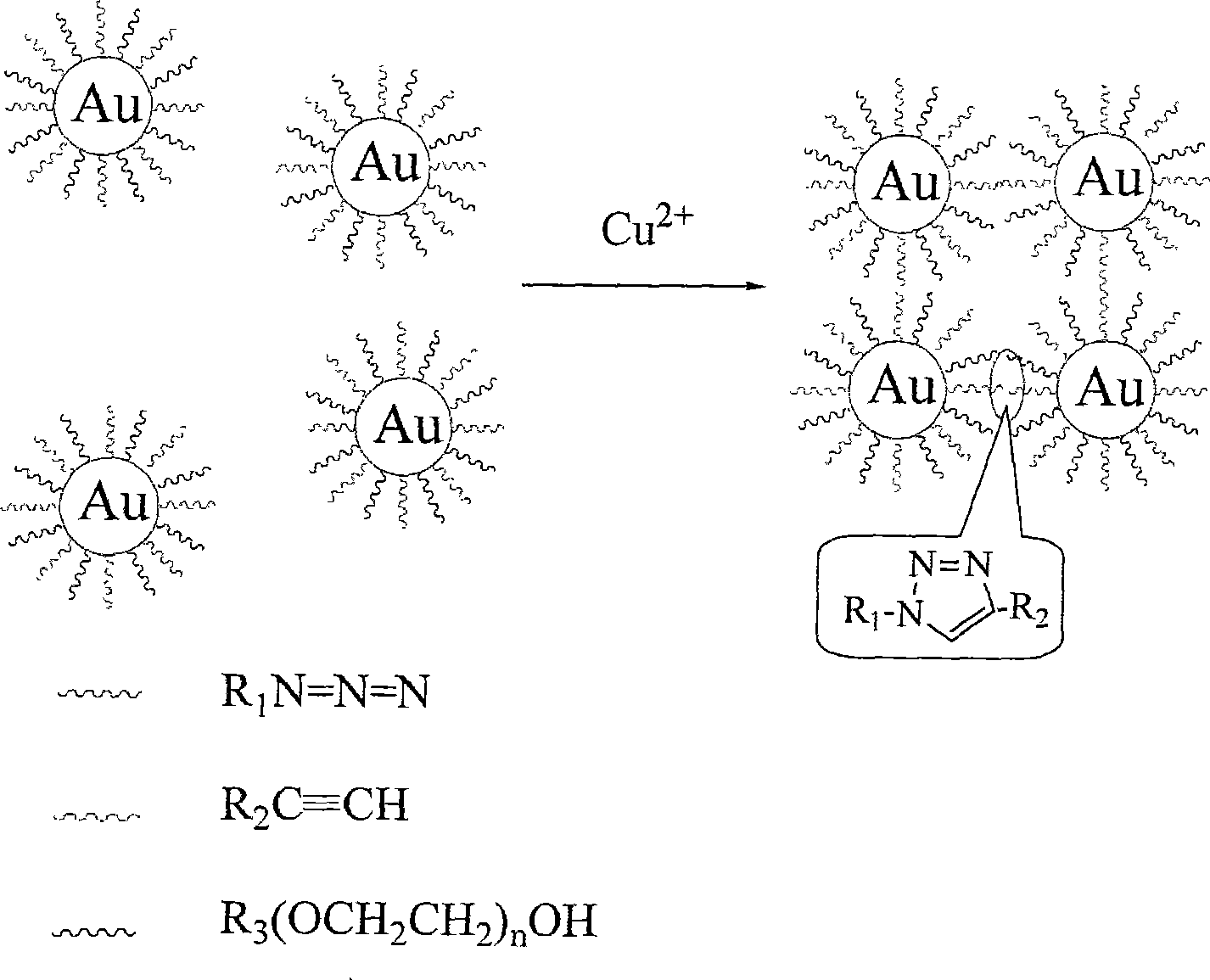
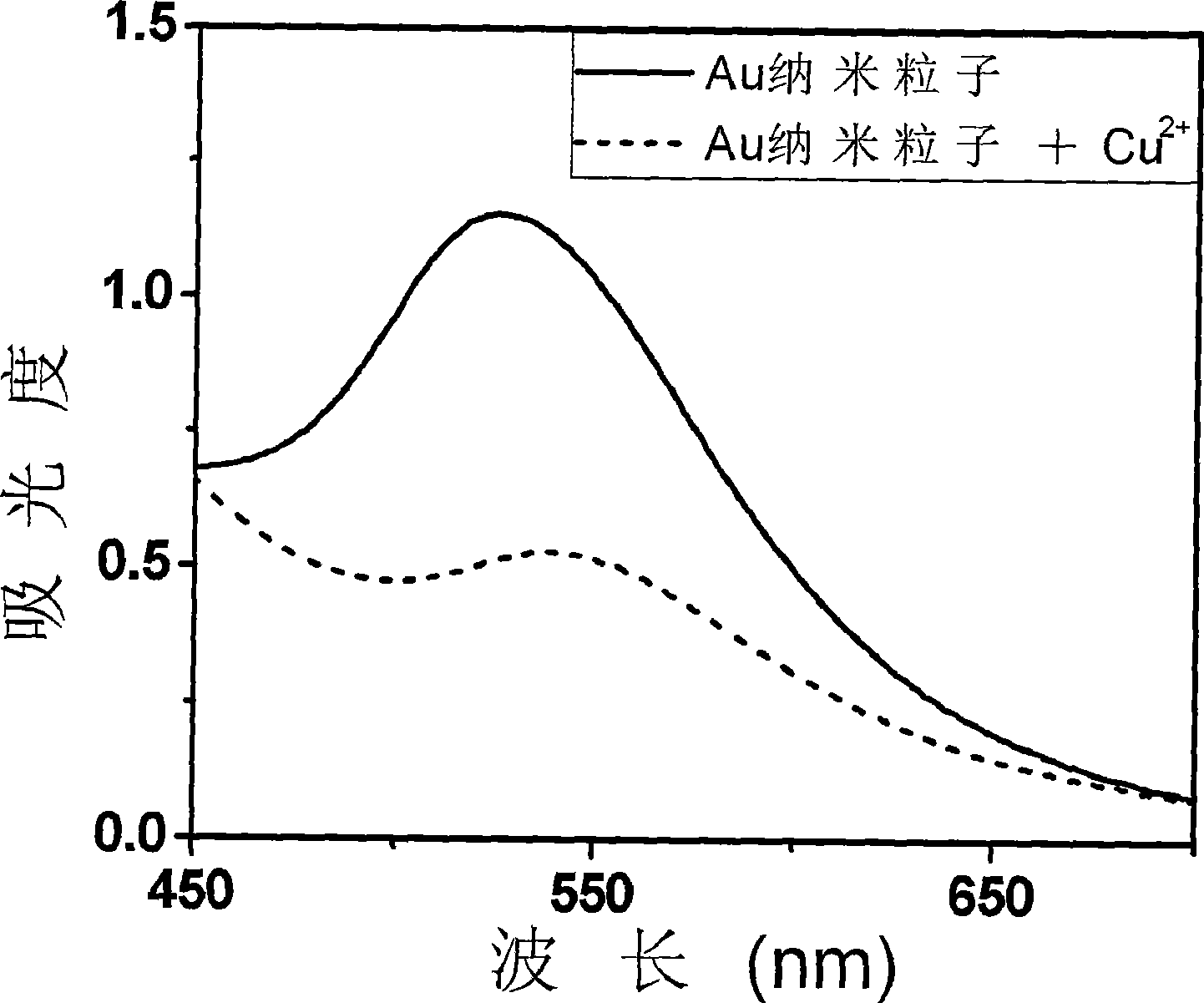
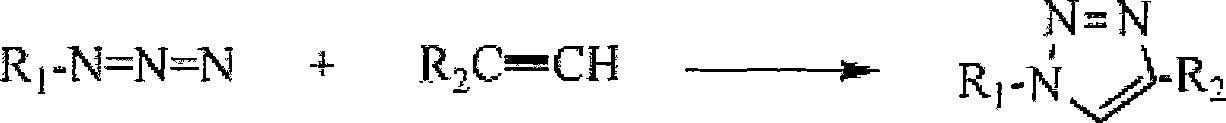Method for qualitatively detecting CU<2+> in solution directly by eye
A qualitative detection and solution technology, which is applied in the direction of material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, etc., to achieve the effects of simple operation, cost saving, and obvious experimental phenomena
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, concrete steps are as follows:
[0034] 1.1: Synthesis of terminal alkyne-functionalized thiols
[0035] 0.5g of 11-alkylthiol carboxylic acid was dissolved in 20mL of anhydrous DMF, then 0.26mL of propyne bromide and 0.1g of anhydrous potassium carbonate were added, and the mixture was stirred at room temperature for 24 hours. The reaction solution was filtered, and DMF was removed by rotary evaporation to obtain 0.593g of crude product; the crude product was further dissolved with 30mL of dichloromethane, washed twice with 30mL of deionized water, and then separated, and the organic phase of dichloromethane was washed with anhydrous magnesium sulfate Drying, rotary evaporation to remove dichloromethane to obtain terminal alkyne functionalized mercaptan 0.475g, its yield was 81%, 1 H-NMR (400MHz, CDCl 3 ): δ2.46(s, 1H), δ2.68(t, J=7.2Hz, 2H), δ2.35(t, J=7.4Hz, 2H), δ1.63~1.28(m, 16H). MS, m / z: 256 (M+1).
[0036] 1.2: Synthesis of terminal azido-funct...
Embodiment 2
[0047] According to the steps of 1.1 and 1.2 in Example 1, a thiol functionalized with a terminal alkynyl group and a terminal azido group was synthesized using a 5-alkylthiol carboxylic acid. 0.05g of 5-alkylthiol carboxylic acid was dissolved in 5mL of anhydrous DMF, then 0.026mL of propyne bromide and 0.01g of anhydrous potassium carbonate were added, and the mixture was stirred at room temperature for 24 hours. The reaction solution was filtered, and DMF was removed by rotary evaporation to obtain the crude product of terminal alkyne functionalized thiol, which was then dissolved in dichloromethane, washed with water, dried over anhydrous magnesium sulfate, and rotary evaporated to obtain the purified terminal alkyne functionalized thiol ;
[0048] Add 0.1mL of chloroethanol and 0.2g of sodium azide to 10mL of DMF, and stir vigorously at 60°C for 48 hours under airtight conditions. After the reaction, wash with water, extract with dichloromethane, dry with anhydrous magnes...
Embodiment 3
[0050] According to the steps of 1.1 and 1.2 in Example 1, a thiol functionalized with a terminal alkynyl group and a terminal azido group was synthesized using a 9-alkylthiol carboxylic acid. 0.1 g of 9-alkylthiol carboxylic acid was dissolved in 10 mL of anhydrous DMF, then 0.1 mL of propyne bromide and 0.05 g of anhydrous potassium carbonate were added, and the mixture was stirred at room temperature for 24 h. The reaction solution was filtered, and DMF was removed by rotary evaporation. The crude product was dissolved in dichloromethane, washed with water, dried over anhydrous magnesium sulfate, and rotary evaporated to obtain terminal alkyne-functionalized mercaptans; 0.2mL chloroethanol and 0.4g sodium azide were added to 10mL In DMF, the reaction was vigorously stirred at 60°C for 48 hours under airtight conditions. After the reaction was completed, it was washed with water, extracted with dichloromethane, dried over anhydrous magnesium sulfate, and rotary evaporated to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com