Chloridization method for nitrogen heterocyclic butanone isobutene derivatives
A technology of azetidinone isobutene and azetidinone isobutene, which is applied in the field of chlorination of azetidinone isobutene derivatives, can solve environmental protection requirements and reduce cost disadvantages, poor selectivity and yield, and olefinic bis Bond displacement and other issues, to achieve the effects of environmental protection restrictions and high recovery rate, fast response, mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
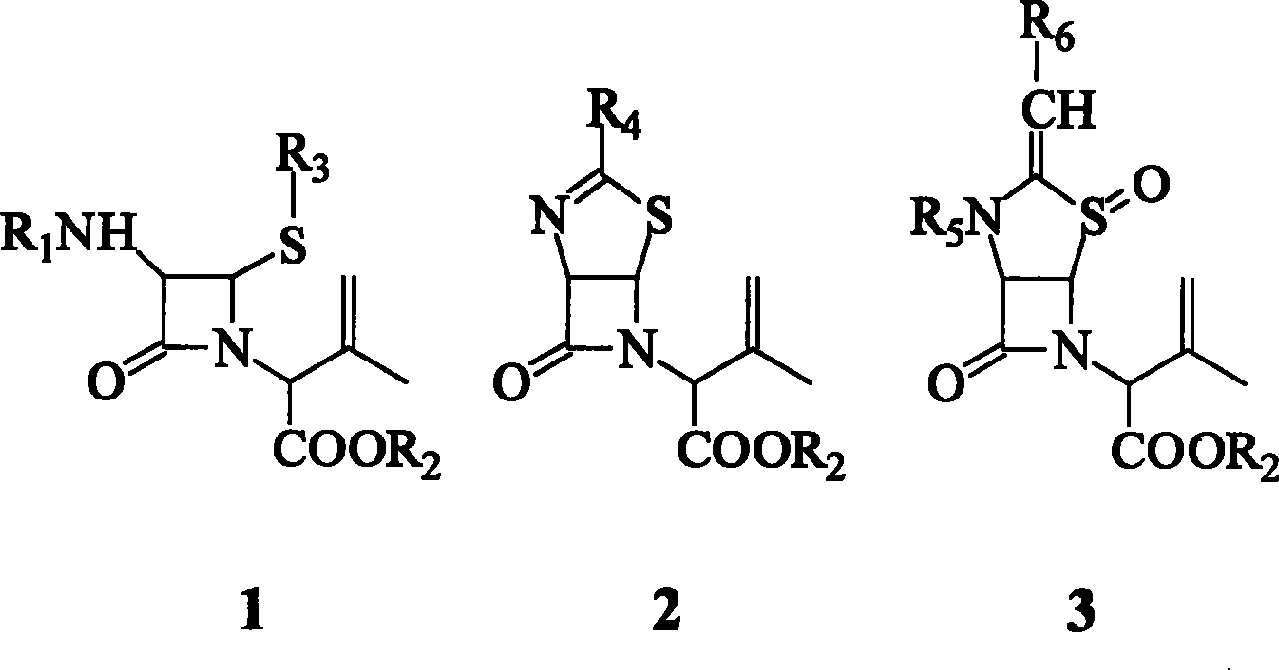
[0021] Take compound 1 (R 1 is phenylacetyl, R 2 p-Methoxybenzyl, R 3 10 millimoles of benzenesulfinic acid group) were dissolved in 100 milliliters of a mixed solution of carbon disulfide, carbon dichloride and propylene oxide (volume ratio 3:2:1), and the temperature was cooled to -5°C with ice brine. In addition, carbon disulfide is used to dissolve chlorine gas, the concentration of the chlorine solution is 1%, and the temperature is controlled at 5° C. with cold water. Under the condition of effective stirring, quickly add the chlorine solution dropwise to the azetidinone isobutene derivative solution to ensure that the reaction temperature is 0°C, the reaction time is 3 minutes, and the molar ratio of the azetidinone isobutene derivative to chlorine gas is 1 :1.3.
[0022] After the dropwise addition, the temperature was kept at 0° C. and stirring was continued for 30 minutes. After the reaction was completed, the solvent was evaporated under reduced pressure in vacu...
Embodiment 2
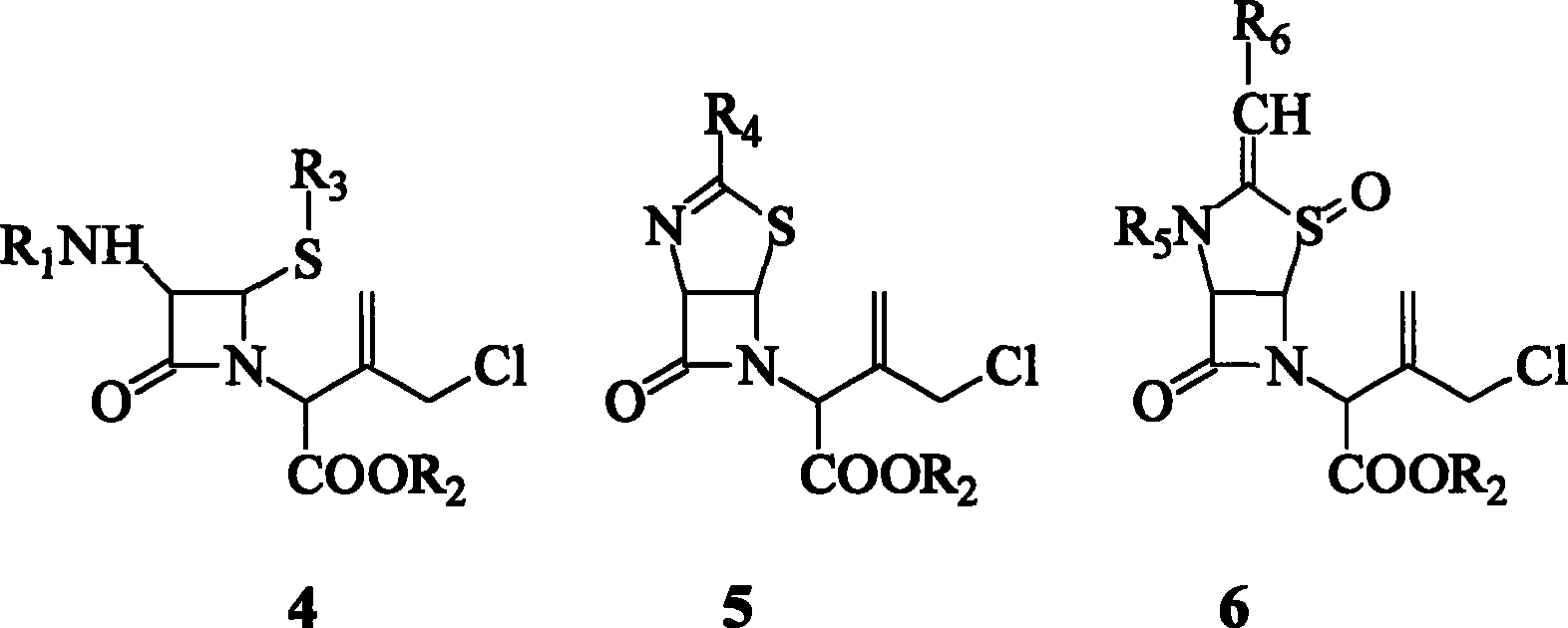
[0027] Take compound 3 (R 2 p-Methoxybenzyl, R 5 is formyl, R6 Benzyl) 10 millimoles were dissolved in 100 milliliters of dioxane, a mixed solution of ethyl acetate and ethylene oxide (volume ratio 3:2:1), and cooled to 10° C. with cold water. In addition, dilute the chlorine gas with helium gas, the concentration of the chlorine gas is 1%, and the temperature of the serpentine coil cooled by cold water is 10°C. Slowly add dilute chlorine gas to the azetidinone isobutene derivative solution under effective stirring, the reaction time is 3 hours, the reaction temperature is guaranteed to be 10°C, and the mol ratio of the azetidinone isobutene derivative to chlorine is 1:1.5.
[0028] After aeration, the temperature was kept at 10° C., and the reaction was continued for 2 hours. After the reaction was completed, the solvent was evaporated under reduced pressure in vacuo to obtain a yellow viscous body, which was the chloride allylic monochloride 6. Place in a refrigerator at...
Embodiment 3
[0033] Take compound 1 (R 1 is phenoxyacetyl, R 2 Methyl, R 3 10 mmoles of 2-mercaptobenzothiazolyl) were dissolved in 100 ml of a mixed solution of carbon dichloride and ethylene oxide (volume ratio 4:1), and cooled to -5°C with ice-cold brine. In addition, carbon dichloride was used to dissolve chlorine gas, the concentration of the chlorine solution was 5%, and the temperature was controlled at -5°C with ice-salt water. Slowly add chlorine solution dropwise to the azetidinone isobutene derivative solution under effective stirring to ensure that the reaction temperature is -5°C, and the molar ratio of the azetidinone isobutene derivative to chlorine gas is 1:1.1.
[0034] After the dropwise addition, the temperature was kept at 0° C., and the reaction was continued for 2 hours. After the reaction was completed, the solvent was distilled off under reduced pressure in vacuo to obtain a yellow viscous body, which was allyl monochloride 4. Place in a refrigerator at 5°C to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com