Process for preparing desogestrel
A technology of desogestrel and its compound, which is applied in the field of preparation of the compound desogestrel, can solve the problems of difficult separation and purification, reduced practical significance, and many reaction by-products, and achieves convenient purification, short reaction route, and improved quality and the effect of the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0054] The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention. Unless otherwise specified, the weight-to-volume ratio mentioned in the examples refers to the ratio of the weight of the solid to the volume of the liquid (1:1 is 1 g of solid to 1 ml of liquid).
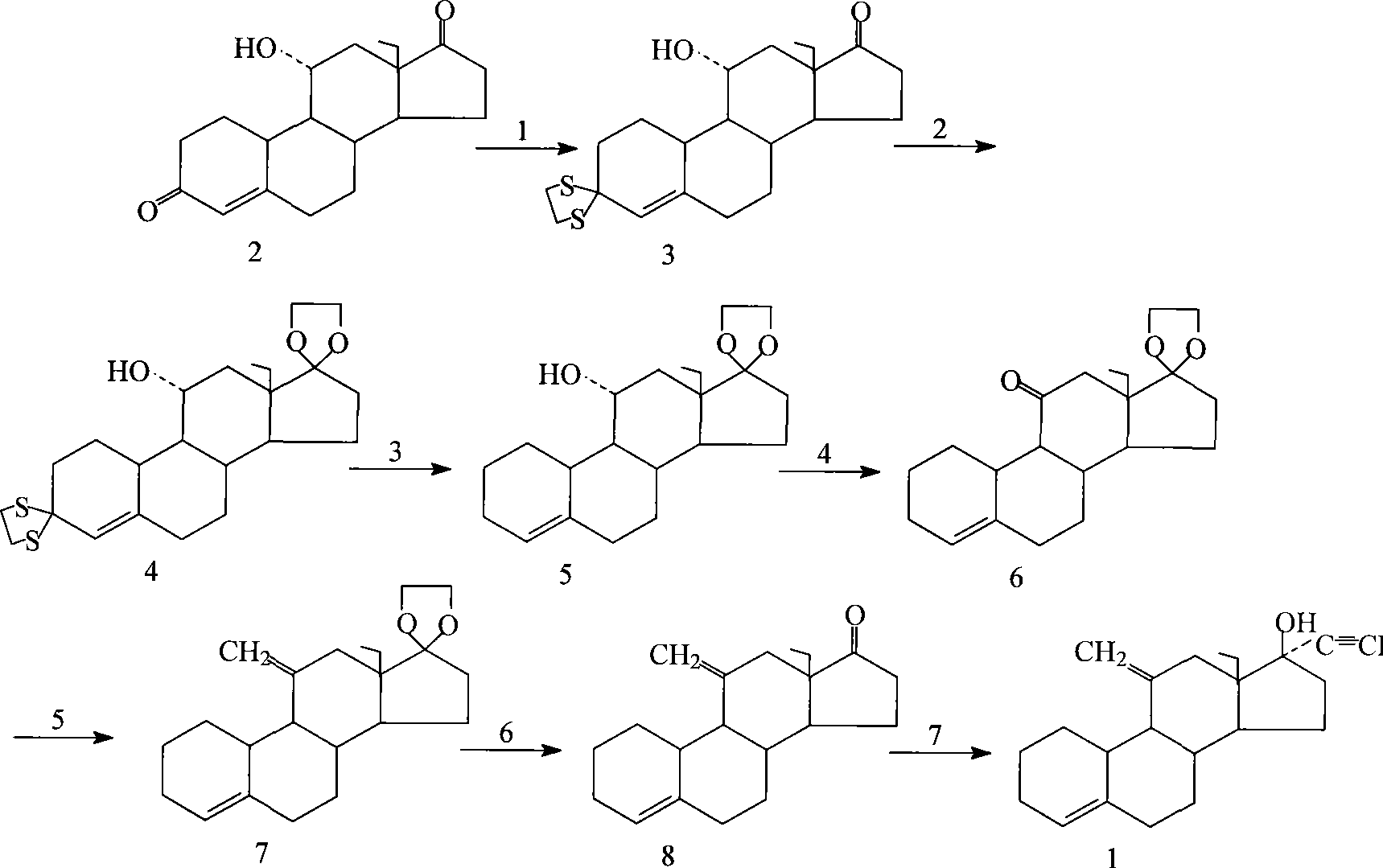
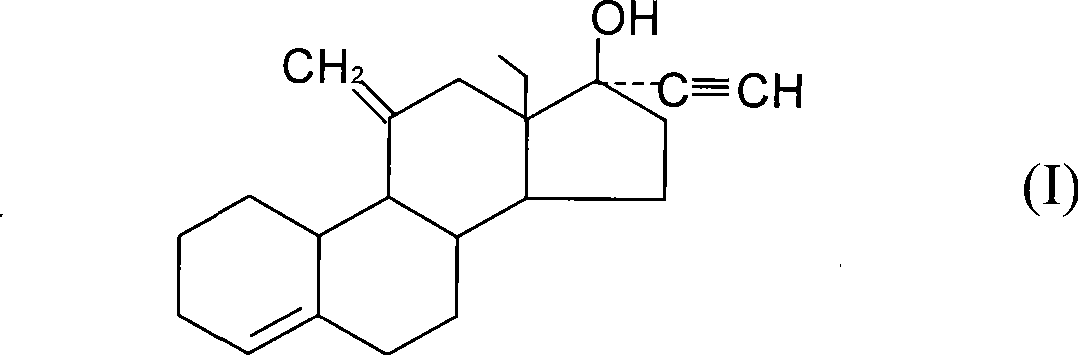
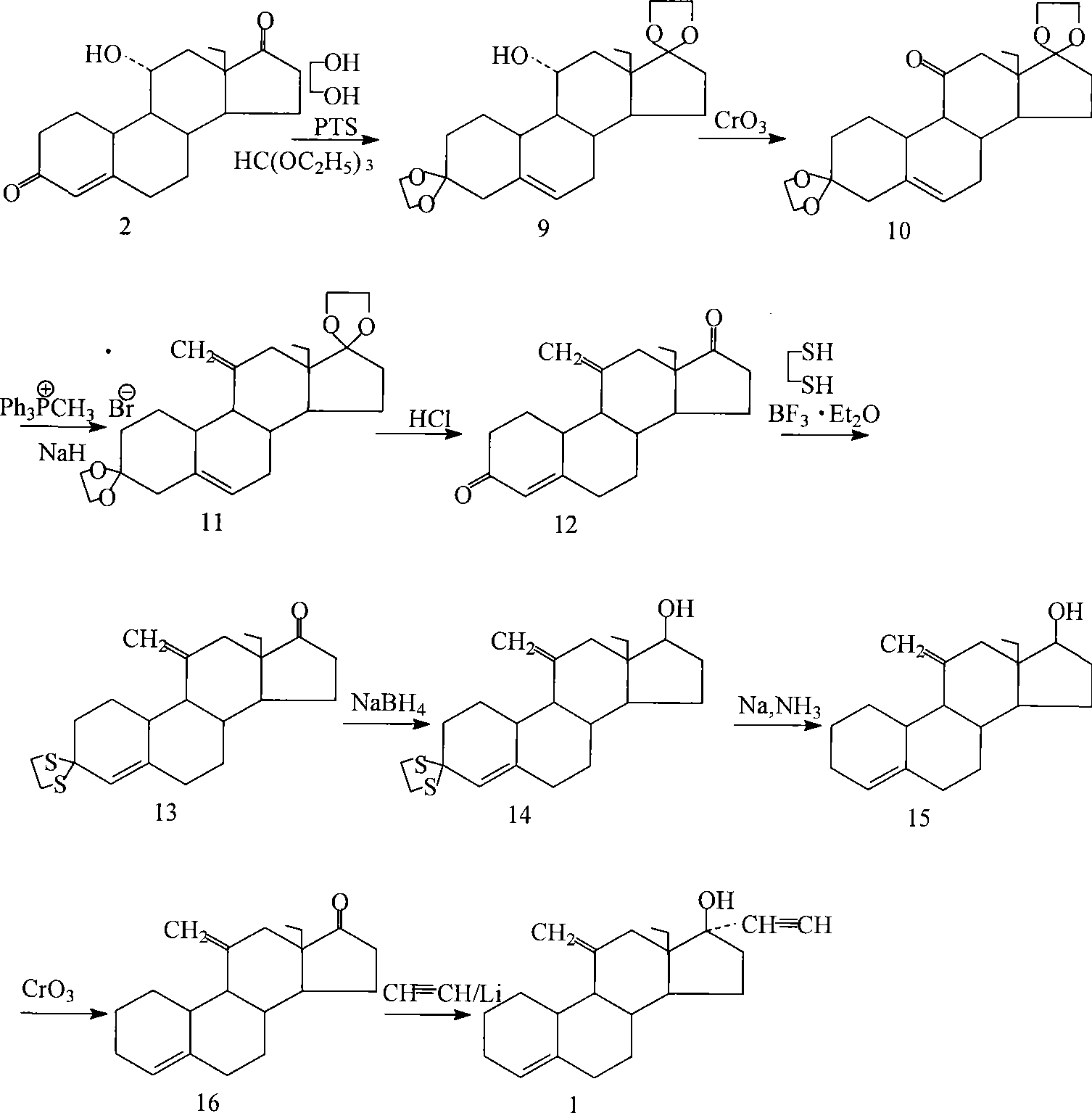
[0055] With 11α-hydroxyl-18-methyl-estr-4-ene-3,17-dione as the starting material (with 18-methyl-estr-4-ene-3,17-dione as the substrate , obtained by transformation with Rhizopus niger (from Institute of Microbiology, Chinese Academy of Sciences. Yield 39%, melting point: 189-192° C., [α]=-10°) followed by the following steps to prepare desogestrel.
[0056] (1) 3,3-ethylene disulfide-11α-hydroxyl-18-methyl-estr-4-en-17-one (compound 3)
[0057] Add glacial acetic acid (75ml) into the reaction vessel, start stirring, put in compound 2 (15g, 49.60mmol) and ethanedithiol (5.0ml, 59.66mmol), adjust the temperature to 25°C, and add 4.5ml of bor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com