GLP-1 derivative
A technology of GLP-1 and its derivatives, which is applied in the field of bioengineering, can solve the problems of loss of activity, etc., and achieve the effect of simple production method, low cost and mature technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
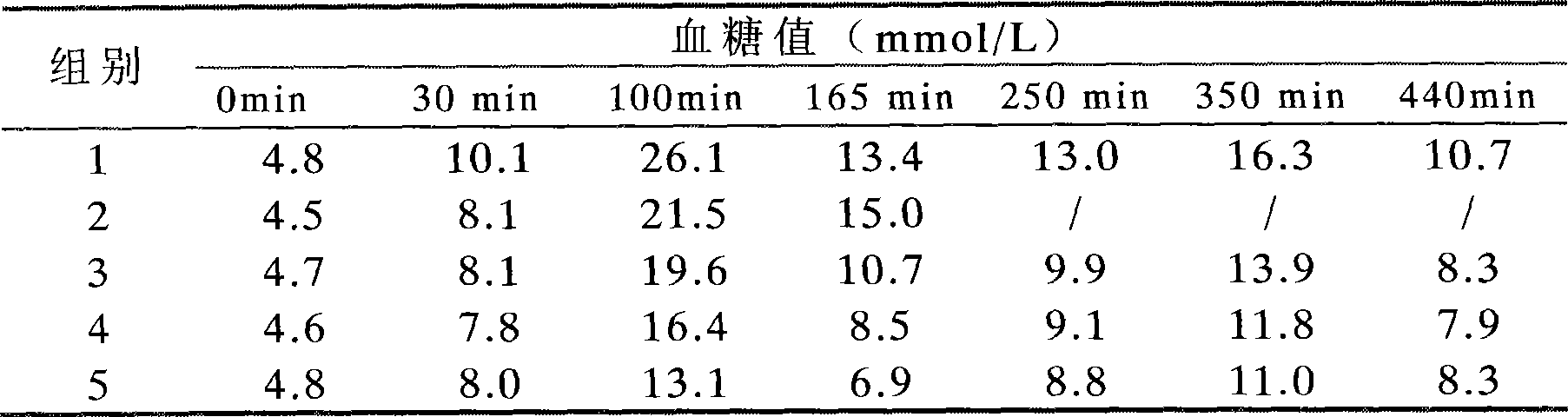
Examples
Embodiment 1
[0037] The GLP-1 derivative of the present invention is synthesized by a solid-phase chemical synthesis method. The molecular structure of the derivative is: aGLP-1(7-36), namely Seq ID No. 2, where Xaa2=D-Ala, Xaa20=Ser, Xaa28=Ala, Xaa30=Cys, and the C-terminal of Cys is amide-NH2, operation steps:
[0038] 1. A total of 16 amino acid monomers with protective groups are used, they are: Fmoc-L-Ala-OH, Fmoc-D-Ala-OH, Fmoc-L-His(Trt)-OH, Fmoc-L-Val -OH, Fmoc-L-Glu(OtBu)-OH, Fmoc-L-Tyr(tBu)-OH, Fmoc-L-Gly-OH, Fmoc-L-Leu-OH, Fmoc-L-Thr(tBu)- OH, Fmoc-L-Gln(Trt)-OH, Fmoc-L-Phe-OH, Fmoc-L-Ser(tBu)-OH, Fmoc-L-Ile-OH, Fnoc-L-Asp(OtBu)-OH , Fmoc-L-Trp-OH, Fmoc-L-Cys(Trt)-OH, where the abbreviation means:
[0039] Fmoc: 9—fluorenyl methoxycarbonyl
[0040] Trt: Trityl, namely trityl
[0041] OtBu: tert-butyl ester
[0042] tBU: tert-butyl, namely tert-butyl;
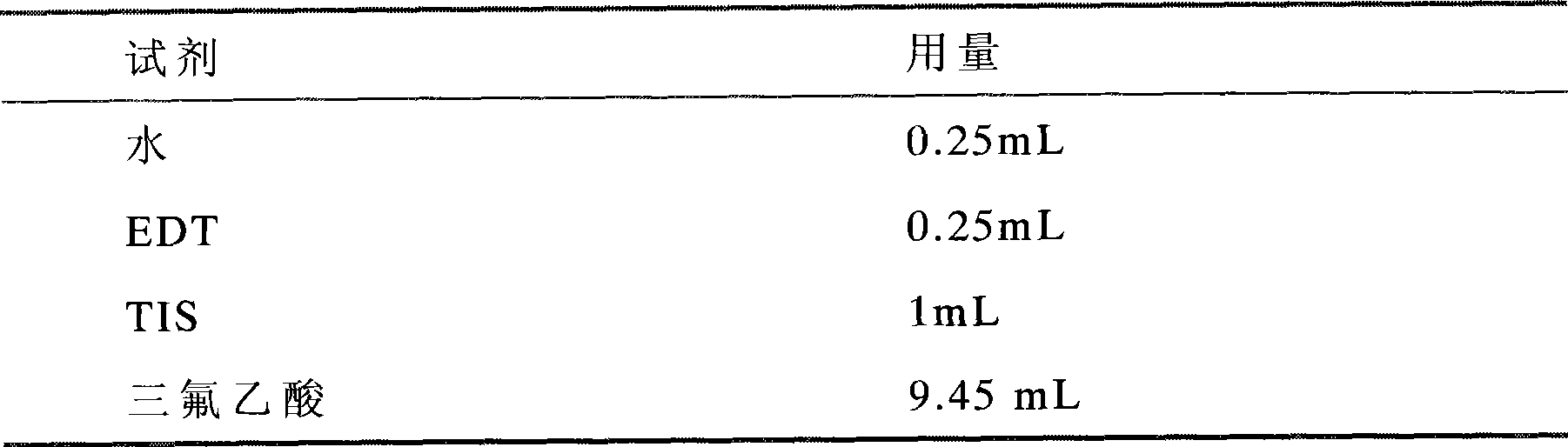
[0043] 2. Equipment and reagents needed
[0044] Instrument: SYMPHONY 12-channel peptide synthesizer, model: SYMPHONY, American prod...
Embodiment 2
[0063] Example 2 The GLP-1 derivative of the present invention is synthesized by solid-phase chemical synthesis. The molecular structure of the derivative is aGLP-1(7-37), namely Seq ID No. 3, where Xaa2=D-Ala, Xaa20= Ser, Xaa28=Ala, Xaa30=Gly, Xaa31=Cys, and the C-terminal of Cys is carboxy-OH. In this example, Wang’s resin is selected.
[0064] In the first step of the operation procedure, weigh 1mmol of the amino acid monomer with the protective group into a bottle, according to the amino acid sequence of the GLP-1 derivative, that is, Seq ID No.3, arranged in SYMPHONY from the C-end to the N-end Type 12-channel peptide synthesizer:
[0065] Fmoc-L-Cys(Trt)-OH, Fmoc-L-Gly-OH, Fmoc-L-Gly-OH, Fmoc-L-Ala-OH, Fmoc-L-Val-OH, Fmoc-L-Leu-OH , Fmoc-L-Trp-OH, Fmoc-L-Ala-OH, Fmoc-L-Ile-OH, Fmoc-L-Phe-OH, Fmoc-L-Glu(OtBu)-OH, Fmoc-L-Ser( tBu)-OH, Fmoc-L-Ala-OH, Fmoc-L-Ala-OH, Fmoc-L-Gln(Trt)-OH, Fmoc-L-Gly-OH, Fmoc-L-Glu(OtBu)-OH , Fmoc-L-Leu-OH, Fmoc-L-Tyr(tBu)-OH, Fmoc-L-Ser(tBu)-OH, Fm...
Embodiment 3
[0067] Example 3 The GLP-1 derivative of the present invention is prepared by a solid-phase chemical synthesis method. The molecular structure of the derivative is aGLP-1(7-38), namely Seq ID No. 4, where Xaa2=Ser, Xaa20=Gln, Xaa28=Asp, Xaa30=Gly, Xaa31=Gly, Xaa32=Cys, and the C-terminal of Cys is carboxy-OH. In this example, Wang’s resin is selected.
[0068] In the first step of the procedure, weigh 1mmol of the amino acid monomer with the protective group into a bottle, and arrange it in SYMPHONY from the C-terminal to the N-terminal according to the amino acid sequence of the GLP-1 derivative, that is, Seq ID No.4. Type 12-channel peptide synthesizer:
[0069]Fmoc-L-Cys(Trt)-OH, Fmoc-L-Gly-OH, Fmoc-L-Gly-OH, Fmoc-L-Gly-OH, Fmoc-L-Asp(OtBu)-OH, Fmoc-L- Val-OH, Fmoc-L-Leu-OH, Fmoc-L-Trp-OH, Fmoc-L-Ala-OH, Fmoc-L-Ile-OH, Fmoc-L-Phe-OH, Fmoc-L-Glu( OtBu)-OH, Fmoc-L-Gln(Trt)-OH, Fmoc-L-Ala-OH, Fmoc-L-Ala-OH, Fmoc-L-Gln(Trt)-OH, Fmoc-L-Gly-OH , Fmoc-L-Glu(OtBu)-OH, Fmoc-L-Leu-OH, Fm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com