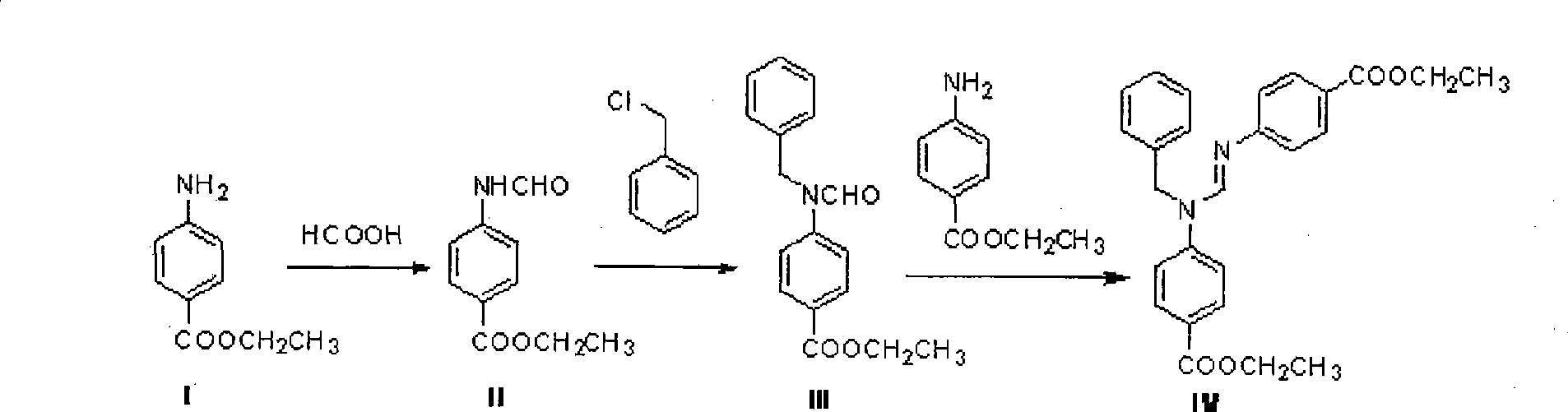
N,N'-bis(4-ethoxy carbonyl phenyl)-N'-benzyl formamidine
A technology of ethoxycarbonyl phenyl and benzyl formamidine is applied in the field of fine chemical industry and achieves the effects of convenient operation, easy separation and purification process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve 50g of ethyl 4-aminobenzoate in 104g of toluene under stirring, then raise the temperature to 43°C, add 18g of formic acid dropwise into the solution, drop it for about 10 minutes, stir for two minutes, then raise the temperature to 100°C to keep the system slightly boiling After about 8 hours, the reaction was stopped, and the reaction solution was slowly cooled to room temperature, and a white solid was precipitated. Suction filtration, washing the solid three times with water, and drying to obtain 56 g of white intermediate ethyl 4-formamidobenzoate with a yield of 96%.
[0022] The resulting intermediate 4-formamidobenzoic acid ethyl ester was dissolved in 233gDMF to obtain a light yellow transparent solution, and 104g K was added thereto 2 CO 3 and 42g of benzyl chloride, then heated up to 60°C, reacted for 23h and cooled to room temperature, poured the reaction solution into water, precipitated a light yellow solid, filtered it with suction, washed the s...
Embodiment 2
[0025] Dissolve 99g of ethyl 4-aminobenzoate in 217g of toluene under stirring, then raise the temperature to 43°C, add dropwise 36g of formic acid into the solution, drop it for about 20 minutes, stir for two minutes, then raise the temperature to 100°C to keep the system slightly boiling After about 10 hours, the reaction was stopped, and the reaction solution was slowly cooled to room temperature, and a white solid was precipitated. Suction filtration, washing the solid three times with water, and drying to obtain 115 g of white intermediate ethyl 4-formamidobenzoate with a yield of 99%.
[0026] Gained intermediate 4-formamidobenzoic acid ethyl ester is dissolved in 378gDMF to obtain light yellow transparent solution, then adds 190g K thereto 2 CO 3 and 79g of benzyl chloride, then heated up to 60°C, reacted for 20h and then cooled to room temperature, poured the reaction solution into water, precipitated a light yellow solid, filtered it with suction, washed the solid wi...
Embodiment 3
[0029] Dissolve 99g of ethyl 4-aminobenzoate in 217g of toluene under stirring, then raise the temperature to 43°C, add dropwise 36g of formic acid into the solution, drop it for about 20 minutes, stir for two minutes, then raise the temperature to 100°C to keep the system slightly boiling After about 10 hours, the reaction was stopped, and the reaction solution was slowly cooled to room temperature, and a white solid was precipitated. Suction filtration, washing the solid three times with water, and drying to obtain 109 g of white intermediate ethyl 4-formamidobenzoate with a yield of 94%.
[0030] Gained intermediate 4-formamidobenzoic acid ethyl ester is dissolved in 378gDMF to obtain light yellow transparent solution, then adds 200g K thereto 2CO 3 and 81g of benzyl chloride, then heated up to 60°C, reacted for 23h and then cooled to room temperature, poured the reaction solution into water, precipitated a light yellow solid, filtered it with suction, washed the solid wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com