Blood platelet additive solution and preparation method thereof
A technology for adding liquid and platelets, applied in the fields of biology and medicine, can solve the problems of high platelet activation rate and poor preservation effect of apheresis platelets, and achieve the effects of preventing aggregation, maintaining preservation quality, and inhibiting activation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation 1 of platelet additive solution (take the preparation of 1000ml as an example):
[0029] Preparation of raw materials:
[0030] Sodium chloride 4.67g; magnesium chloride 0.41g; potassium chloride 0.38g; sodium citrate 4.41g; sodium acetate 2.05g; glucose 2.97g; sodium dihydrogen phosphate 0.63g; sodium bicarbonate 1.09g; L-arginine 30mg; (the above raw materials are pharmacopoeia grade, the same below), sufficient double distilled water.
[0031] One 1000ml container, one measuring cup, one glass stirring rod, one 1000ml sterile medical plastic bag, one 0.22μm sterile filter.
[0032] Preparation Process:
[0033] The ambient temperature is 21°C.
[0034] Put the pharmacopoeia-grade drug into a container, add 800ml of double-distilled water, stir until the color becomes clear, continue to add double-distilled water to adjust to 1000ml, stir evenly to form a medicinal liquid; filter the medicinal liquid with a 0.22 μm sterile filter To sterility, obtain 100...
Embodiment 2
[0036] Preparation 2 of platelet additive solution (take the preparation of 1000ml as an example):
[0037] Preparation of raw materials:
[0038] Sodium chloride 4.95g; magnesium chloride 0.38g; potassium chloride 0.41g; sodium citrate 4.21g; sodium acetate 2.15g; glucose 3.60g; sodium dihydrogen phosphate 0.57g; sodium bicarbonate 0.98g; L-arginine 25mg; enough double distilled water.
[0039] One 1000ml container, one 500ml container, one measuring cup, one glass stirring rod, one 1000ml sterile medical plastic bag, one 100ml sterile medical plastic bag, and one flexible packaging ventilated dry heat sterilizer.
[0040] Preparation Process:
[0041] The ambient temperature is 24°C.
[0042] Put sodium chloride, magnesium chloride, potassium chloride, sodium citrate, sodium acetate, sodium bicarbonate, and L-arginine in the raw materials into a 1000ml container, add 800ml double distilled water, and continue to add double distilled water after dissolving Adjust the dist...
Embodiment 3
[0044] Application of the platelet additive solution obtained in Example 1 in the collection of concentrated platelets from multiple people
[0045] Implementation process:
[0046] Separation of the buffy coat (BC) layer of a single blood unit: take 400ml of raw whole blood collected in a quadruple bag, centrifuge at a centrifugal force of 3450g for 10min in a centrifuge at a temperature of 22°C±2°C, and centrifuge the platelet-rich The buffy coat layer (containing platelets, red blood cells, and white blood cells) is extruded into a sub-bag, and the extruded volume is 40ml to 45ml. After heat sealing, it is separated from the mother bag, and the extruded buffy coat is left standing overnight at 22°C±2°C. .
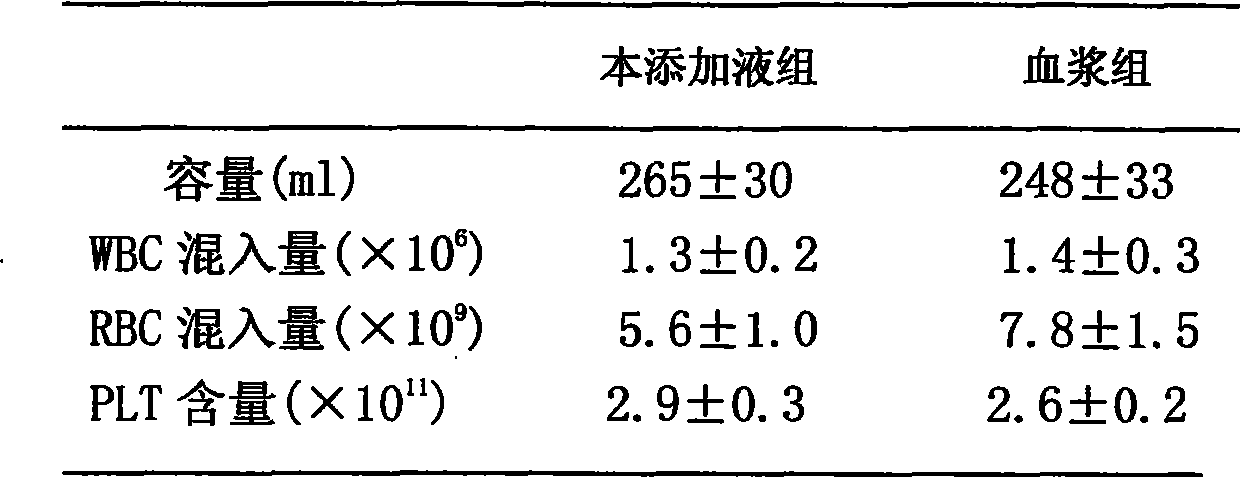
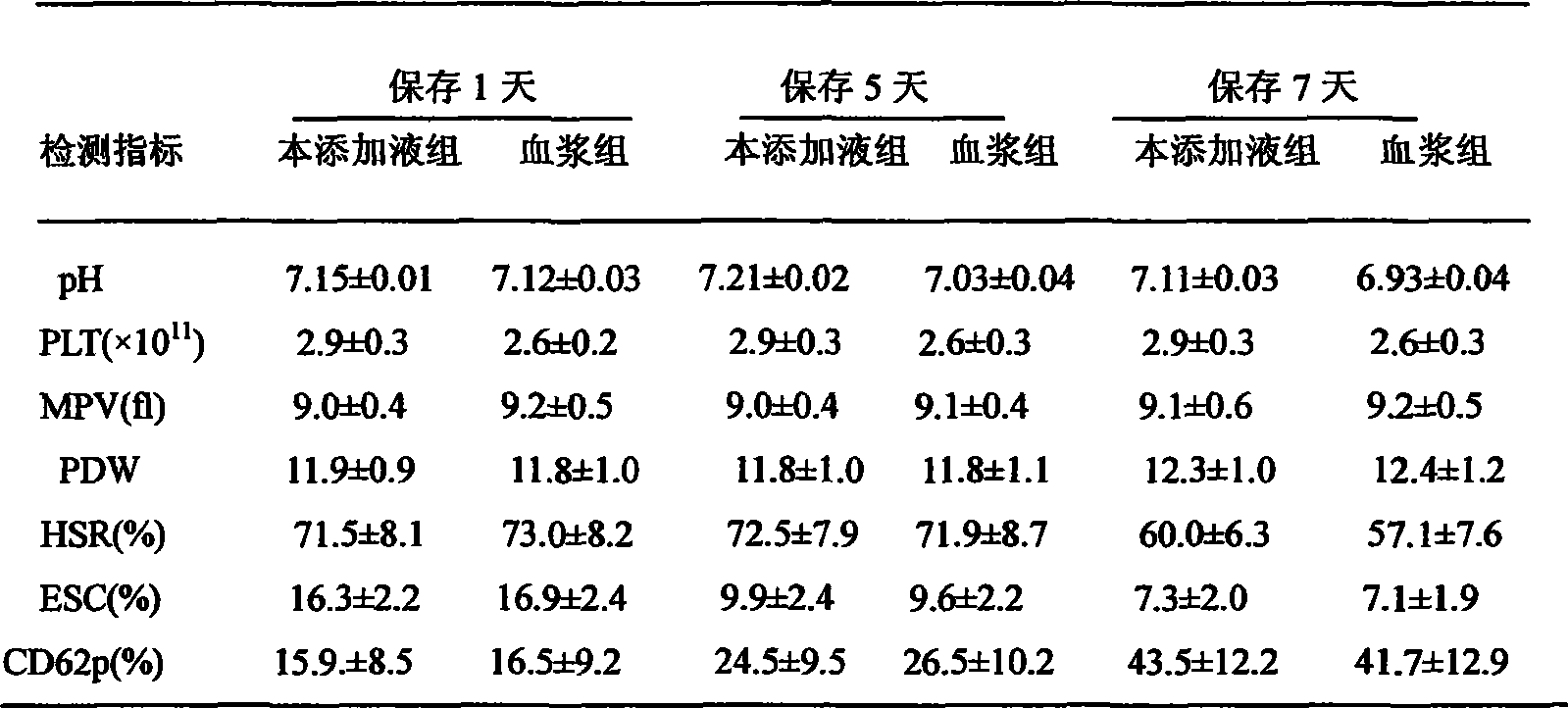
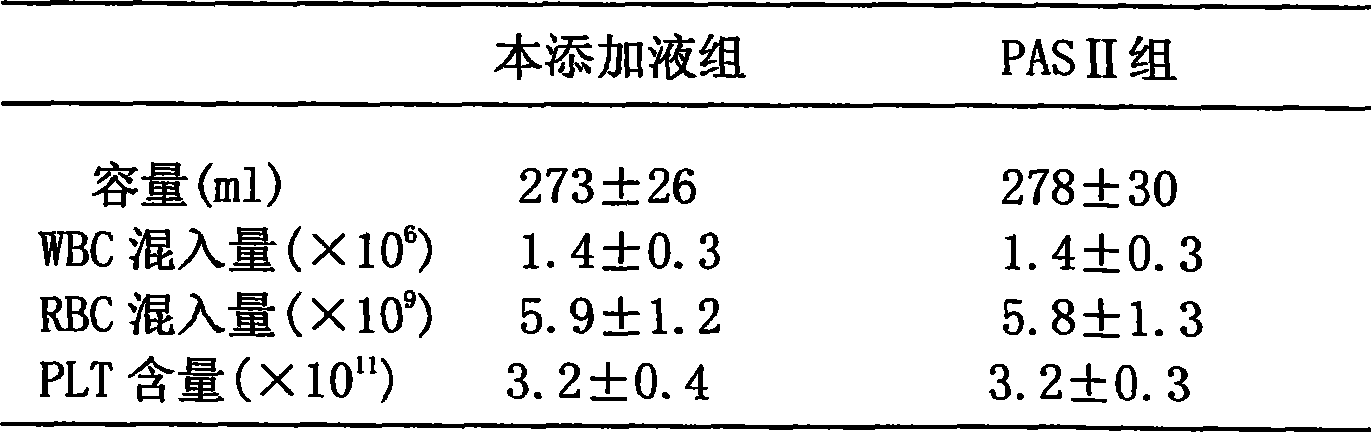
[0047] Adding liquid to collect the buffy coat layer to prepare multiple people’s concentrated platelets: collect 6 bags of buffy coat with the same ABO and RH blood types into the same plasma bag with sterile connection technology, and then add 200-220ml of platelet ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com