Alkaline protease
A protease and alkaline technology, applied in the field of alkaline protease, can solve the problems of low hydrolysis rate and achieve high hydrolysis efficiency, high degree of hydrolysis, and strong adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 Fermentation of hair enzyme and extraction of crude enzyme
[0028] 1.1 Strains
[0029] Mucor actinosa AS3.2778 was purchased from the General Microbiology Center of China Committee for the Collection of Microorganisms.
[0030] 1.2 Seed medium and seed activation
[0031] Add 10g of bran and 10ml of water into a 250ml triangular flask, stir and mix evenly with a glass rod, and smash larger agglomerates (try to make the particles uniform). Sterilize at 121°C for 30 minutes, take it out from the heat, shake it up, and cool it down for later use.
[0032] The sterilized seed medium was inoculated with a ring of slant spores in a test tube per bottle, mixed evenly, and the surface of the medium was made as smooth as possible, and the thickness of the medium layer was consistent, and then placed in a biochemical incubator at 28°C for 3 days.
[0033] 1.3 Preparation of spore suspension
[0034] Under sterile conditions, add 100 ml of sterile water to the a...
Embodiment 2
[0043] The separation and purification of embodiment 2 alkaline protease
[0044] 2.1 Sectional salting-out of ammonium sulfate
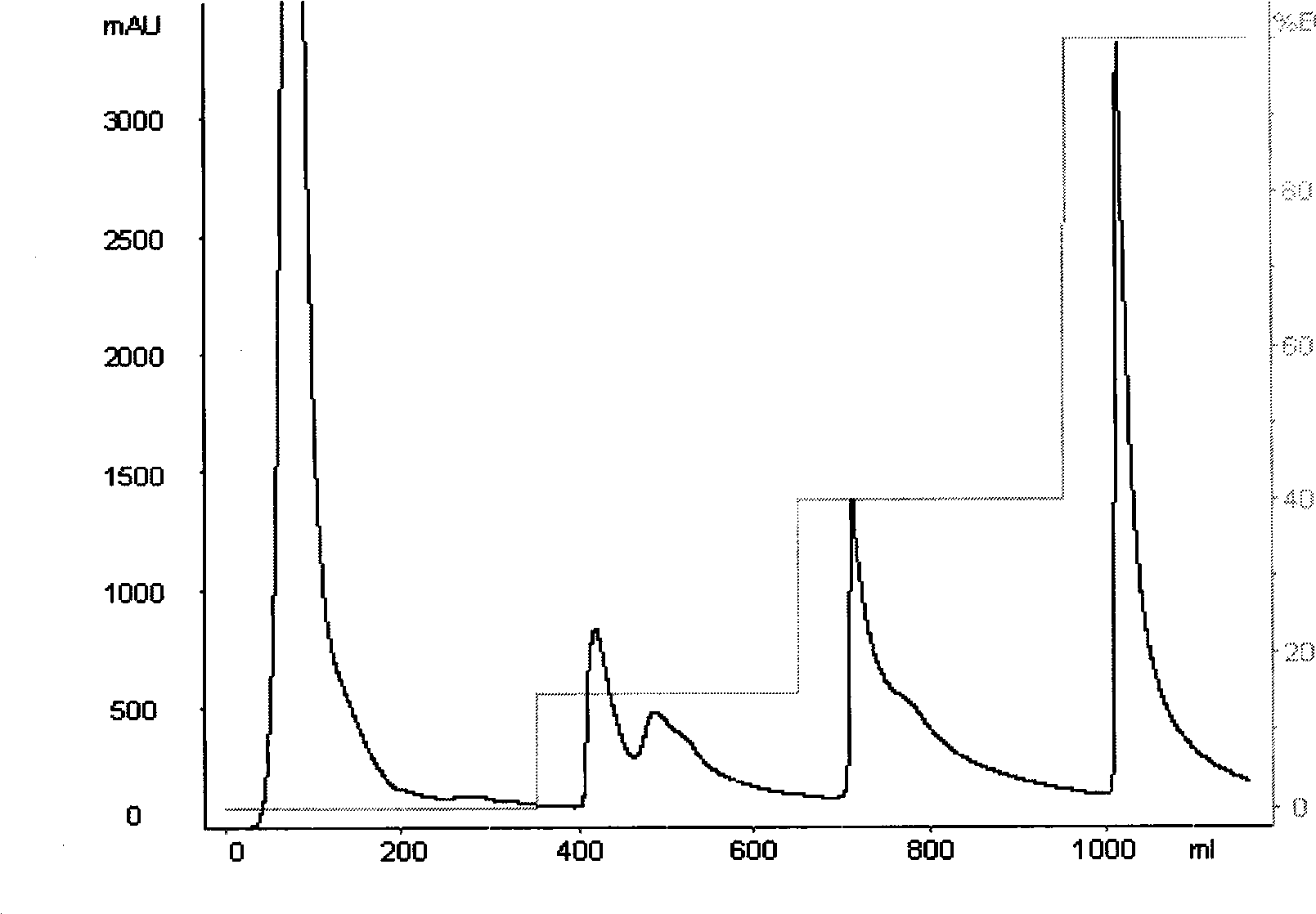
[0045] Take a certain volume of crude enzyme solution, slowly add solid ammonium sulfate to 40% saturation on an ice-water bath, fully dissolve and let stand in a refrigerator at 4°C for 3 hours, centrifuge at 12000g / min for 20min at 4°C, take the supernatant and continue Add solid ammonium sulfate to 85% saturation, let it stand overnight in a refrigerator at 4°C, then centrifuge at 12000g / min for 20min at 4°C, dissolve the protein precipitate with 0.02M, pH7.5 Tris-HCl buffer, and dialyze for desalting.
[0046]2.2 DEAE-Sepharose anion exchange
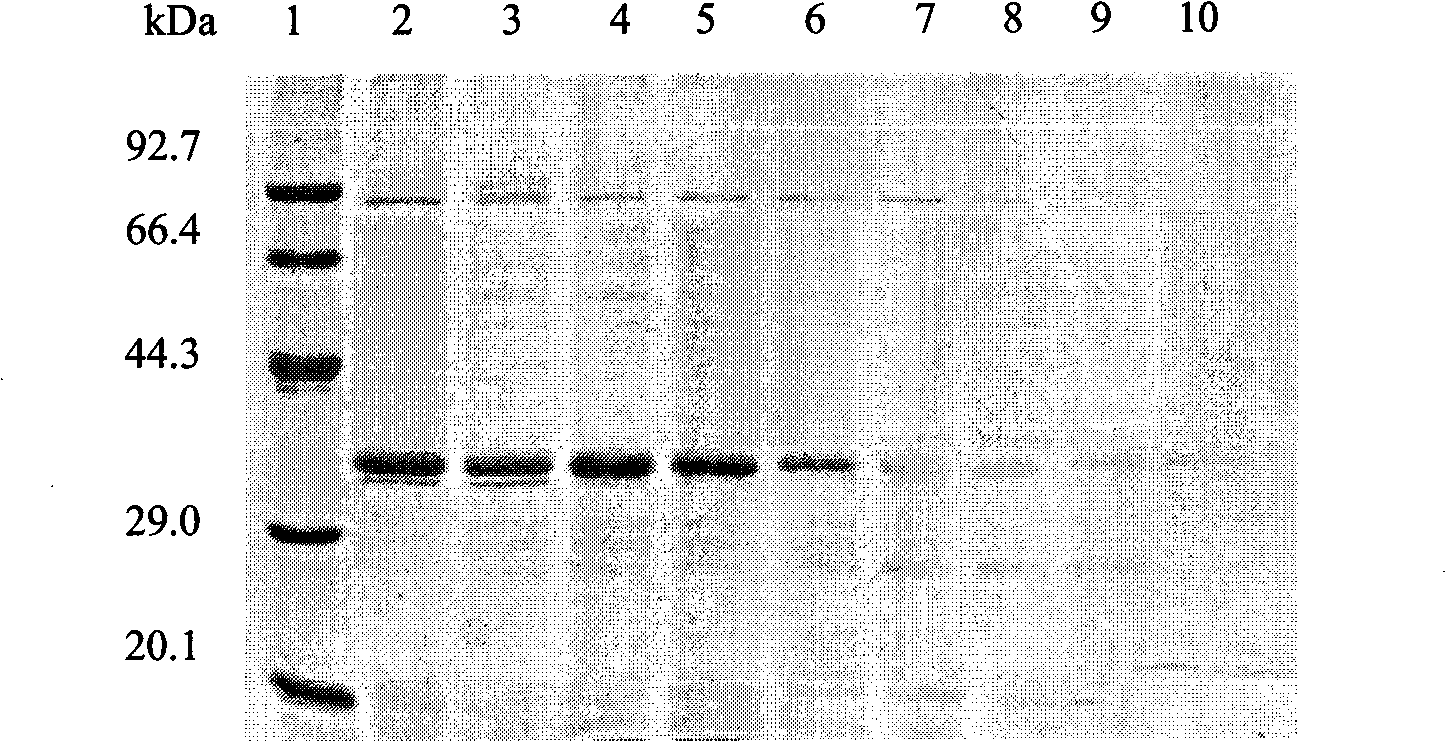
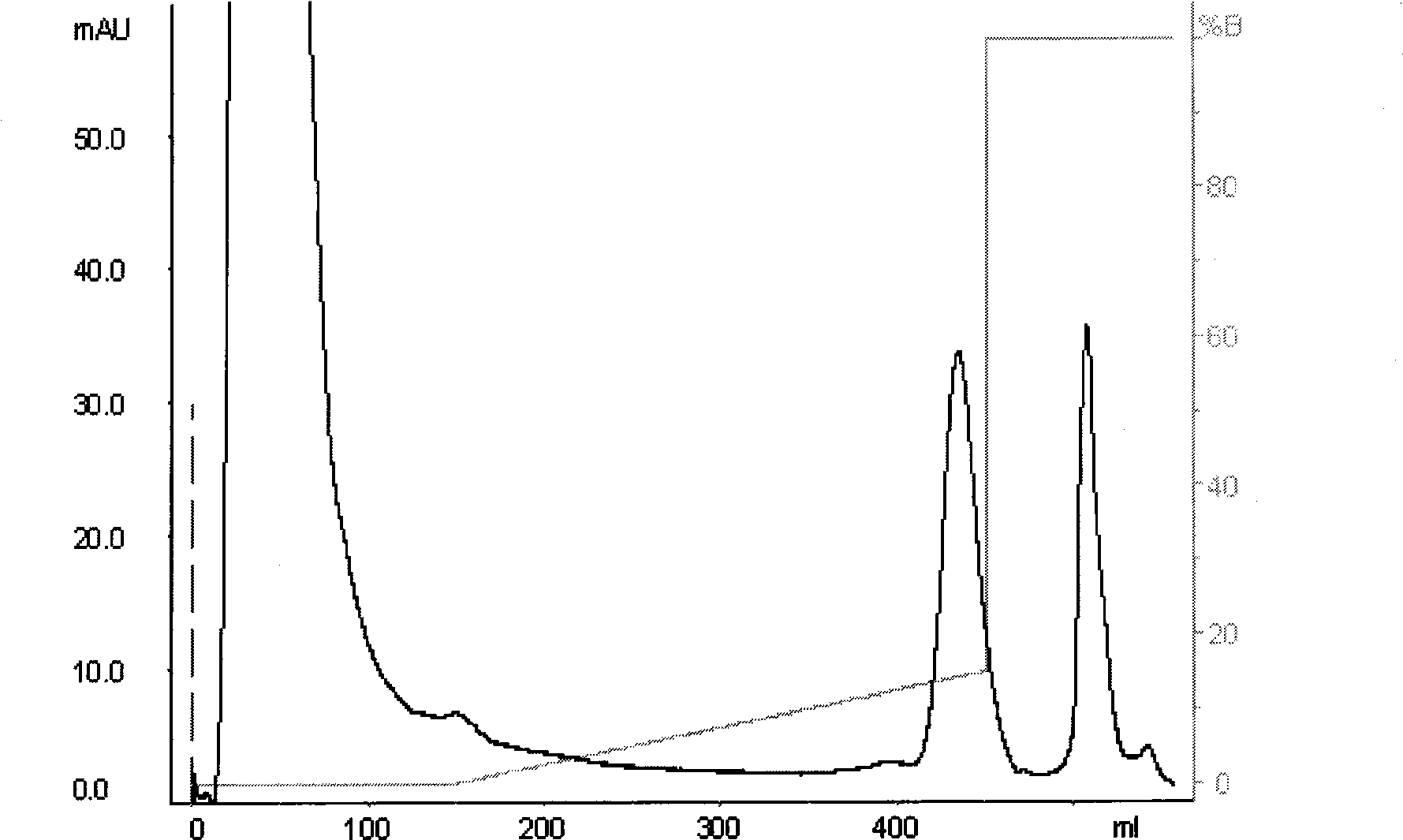
[0047] Equilibrate the DEAE-Sepharose anion-exchange column with 0.02M, pH7.5 Tris-HCl buffer solution (the basis for good balance: the effluent pH7.5, the conductivity is stable), take a certain amount of enzyme solution after salting out and desalting, and add the sample anion exchange column. With 0...
Embodiment 3
[0066] Example 3 Determination of Catalytic Properties of Mucor Alkaline Protease Pb1
[0067] 3.1 Optimal action temperature of alkaline protease
[0068] According to the enzyme activity assay method, the activity of Mucor alkaline protease was measured at different temperatures (range of 30-70° C.), and the effect of temperature on the activity of Mucor alkaline protease was investigated. According to the experimental results, the temperature-activity curve was drawn, and the optimum action temperature of mucoralline protease was determined accordingly.
[0069] 3.2 The optimal pH of mucor alkaline protease
[0070] According to the enzyme activity assay method, under different pH buffer conditions (pH 3.0-5.0, 0.05M acetic acid buffered saline; pH6.0-7.0, 0.05M phosphate buffered saline; pH 8.0-9.0, 0.02M Tris-HCl; pH 9.5- 10.5, 0.02M glycine-NaOH) to measure the activity of Mucor alkaline protease, and investigate the effect of pH on the activity of Mucor alkaline prote...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com