Method for producing benzoic acid
A production method and technology of benzoic acid, which are applied in the fields of hydrocarbon liquid phase oxidation and rectification separation, can solve the problems of high energy consumption, long reaction time, and low product yield, and achieve reduced toluene unit consumption, shortened reaction time, and refined The effect of shortening the distillation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
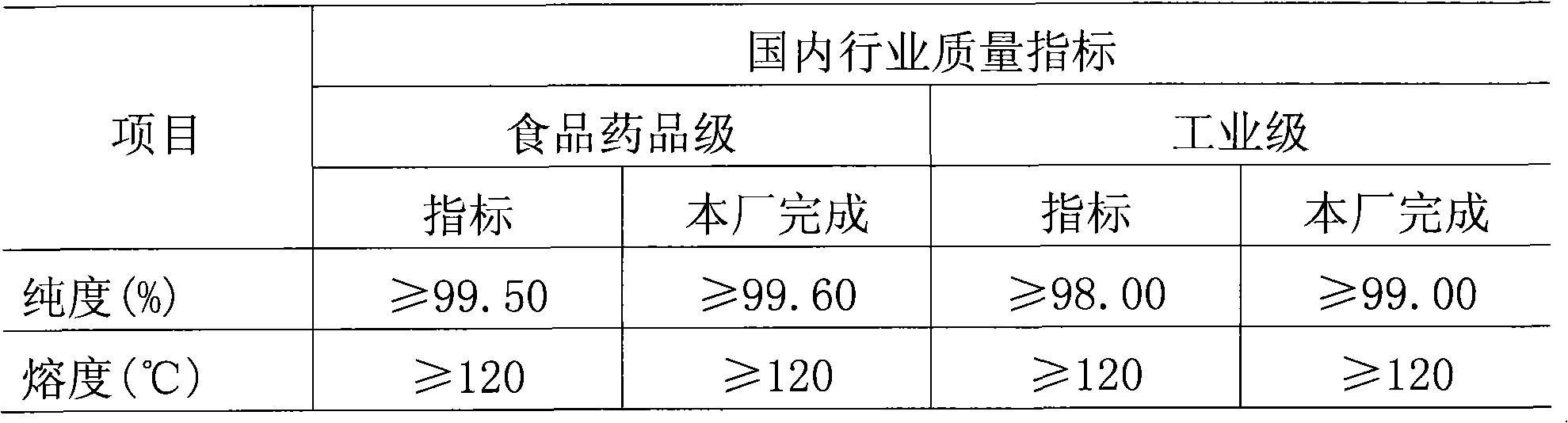
[0015] Each batch of raw material toluene was put into 10 tons, and the catalyst was put into 20 kg of cobalt naphthenate. The oxidation reaction time was 10 hours, the reaction temperature was 165°C, and the reaction pressure was 0.58Mpa. When the oxidation reaction was stopped for 10 hours, the oxidation tail gas was sampled and analyzed. In the tail gas, O 2 The content is 20.180%, the reaction is complete, and the conversion rate is 78%.
Embodiment 2
[0017] Each batch of raw material toluene was put into 12 tons, and 24 kg of catalyst cobalt naphthenate was put in. The oxidation reaction time was 9 hours, the reaction temperature was 160°C, and the reaction pressure was 0.60Mpa. When the oxidation reaction was stopped for 9 hours, the oxidation tail gas was sampled and analyzed, and O in the tail gas 2 The content is 20.517%, the reaction is complete, and the conversion rate is 82%.
Embodiment 3
[0019] Each batch of raw material toluene was put into 12 tons, and 24 kg of catalyst cobalt naphthenate was put in. The reaction temperature was 160°C, the reaction pressure was 0.59Mpa, and the reaction time was 8 hours. When the oxidation reaction was stopped for 8 hours, the oxidation tail gas was sampled and analyzed. In the tail gas, O 2 The content is 17.28%, the reaction is not complete, and the conversion rate is 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com