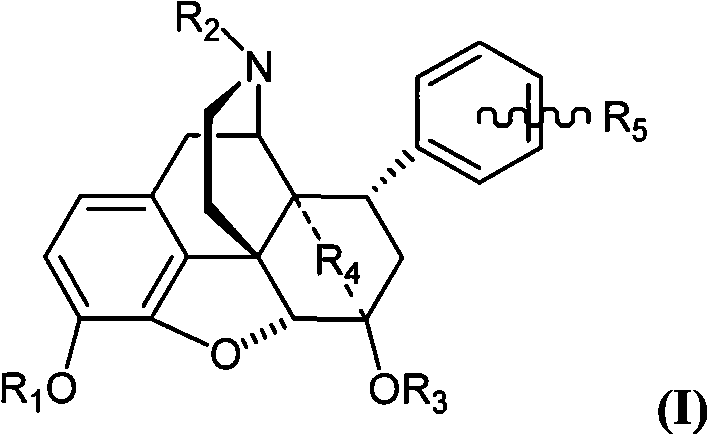
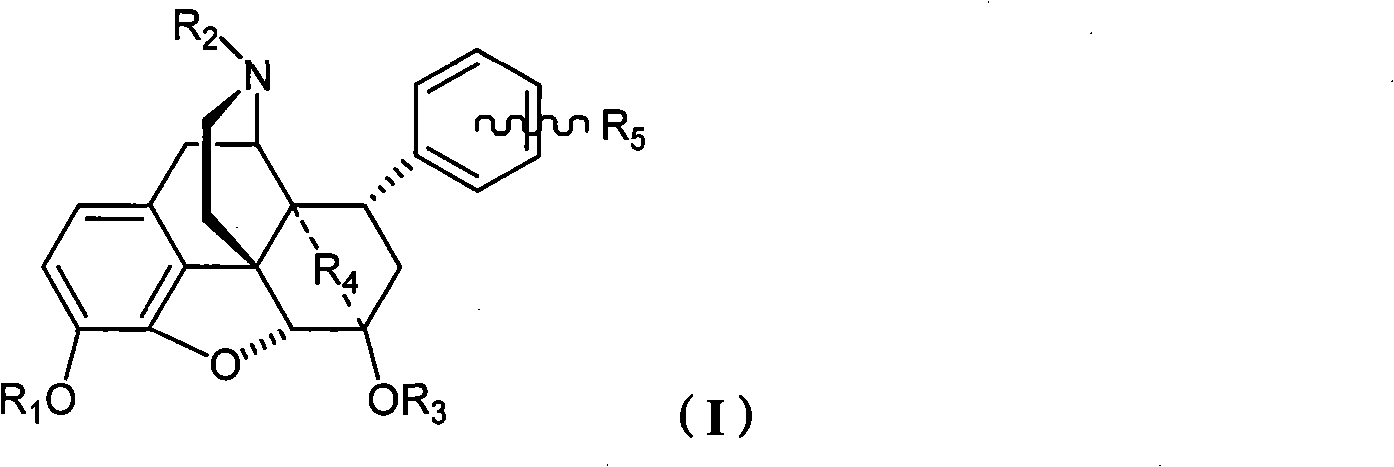
8alpha substituted aryl-4,5-epoxy morphinan derivative or its salt, preparation method and application thereof
A technology of epoxymorphine and derivatives, applied in the field of use in the treatment of pain, can solve the problems such as no 8α-substituted compounds have been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
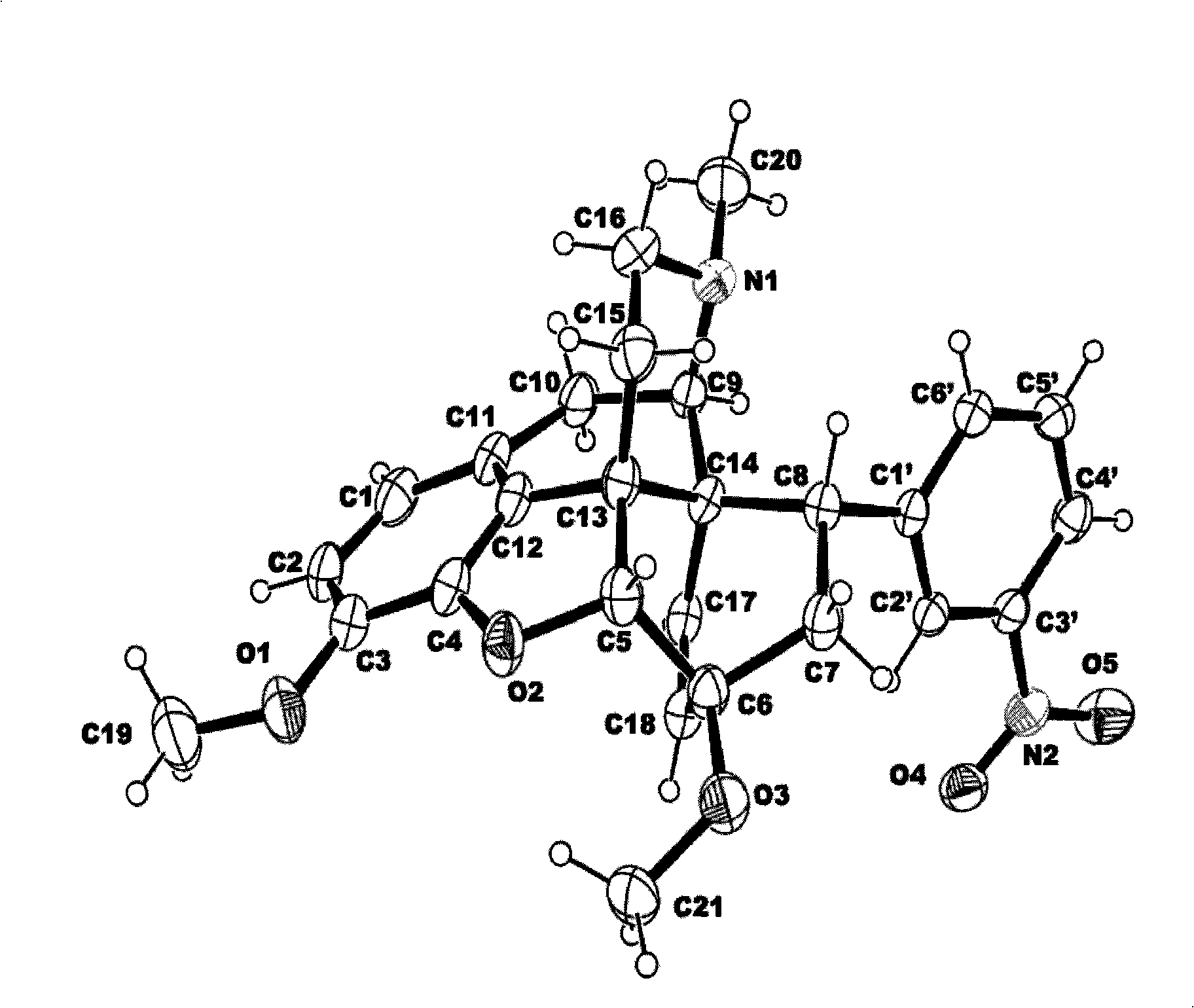
[0031] Preparation of 8α-(3'-nitro-phenyl)-6α, 14α-endo-vinyl-tetrahydrothebaine (R in the same method can be prepared in formula (I) 5 == NO 2 , halogen, cyano or CF 3 Derivatives)
[0032] Add 15g (0.0482mol) of thebaine, 21.6g (0.145mol) of m-nitrostyrene and 70mL of xylene into a 150mL three-necked flask with magnetic stirring, reflux condenser and thermometer. The reaction was refluxed for 24 hours. After the reaction was completed, it was concentrated to dryness under reduced pressure to obtain 53 g of a brown-yellow oil. Silica gel column chromatography, gradient elution with 10%-25% ethyl acetate / petroleum ether solution, gave a white solid with a yield of 2.7%, m.p.157-159°C. Single crystal culture: Add 20 mL of ethyl acetate to about 300 mg of 8α-(3'-nitro-phenyl)-6α,14α-endo-vinyl-tetrahydrothebaine obtained by silica gel column chromatography, and heat to dissolve . Use a 10mL glass syringe to draw 10mL of the solution, press filter through a microporous membr...
Embodiment 2
[0033] Example 2 Culture of CHO cells stably expressing opioid receptors and preparation of crude extracts of opioid receptors
[0034] CHO cell lines stably expressing human κ, rat μ or rat δ opioid receptors were cultured in F12 medium containing 10% newborn bovine serum and 0.25 mg / mL G418, and contained 5% CO 2 , and incubated under 95% humidified air conditions. Measuring the affinity of candidate compounds for opioid receptors or measuring [ 35 S] When GTPγS is bound, cells are seeded to 175 cm 3 On the culture plate, when it grows to 90% confluence, the cells are eluted with a phosphate buffer solution containing 1 mM EDTA, and centrifuged at 1000 rpm for 10 min. The obtained cell pellet was suspended in a solution containing 50mM HEPES, 3mM MgCl 2 and 1 mM EGTA in ice-cold homogenization buffer at pH = 7.4. And use the Dounce homogenizer made of glass to grind 10 times, after homogenization, centrifuge at 18000rpm for 10min (at 4°C), the sediment is treated with h...
Embodiment 3
[0035]Example 3 Determination of the Affinity of Candidate Compounds to Opioid Receptors in Vitro (Radioligand Binding Experiment)
[0036] In the binding experiment, a total binding group, a non-specific binding group and a sample group to be tested were set up. Add approximately 30 μg of membrane receptors to the total binding group, [ 3 H] Diprenorphine (0.5 nM final concentration), adjusted to a final volume of 200 μL with 50 mM Tris-HCl (pH=7.4). For the corresponding non-specific binding group, 1 μM Naloxone was added, and for the test sample group, different concentrations of the test compound or morphine were added, and the final volume was adjusted to 200 μL with 50 mM Tris-HCl (pH=7.4). Water bath at 37°C for 30 min, then put in an ice bath to terminate the reaction. Suction filtration through GF / C (Whatman) glass fiber filter paper on the Millipore sample collector. Rinse the filter paper three times with ice-cold 50mM Tris-HCl (pH=7.4), 4ml each time, dry the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com