Method for detecting eedysterone in medicine composition
A technology of ecdysterone and composition, which is applied in the direction of drug combination, pharmaceutical formula, measuring device, etc., and can solve the problems of difficulty in controlling the quality of finished products, large errors in content determination results, and poor reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] A Houyanqing oral liquid produced by the applicant company was used as a test sample, which was also made from the aforementioned four Chinese herbal medicines. The method of content determination is as follows:
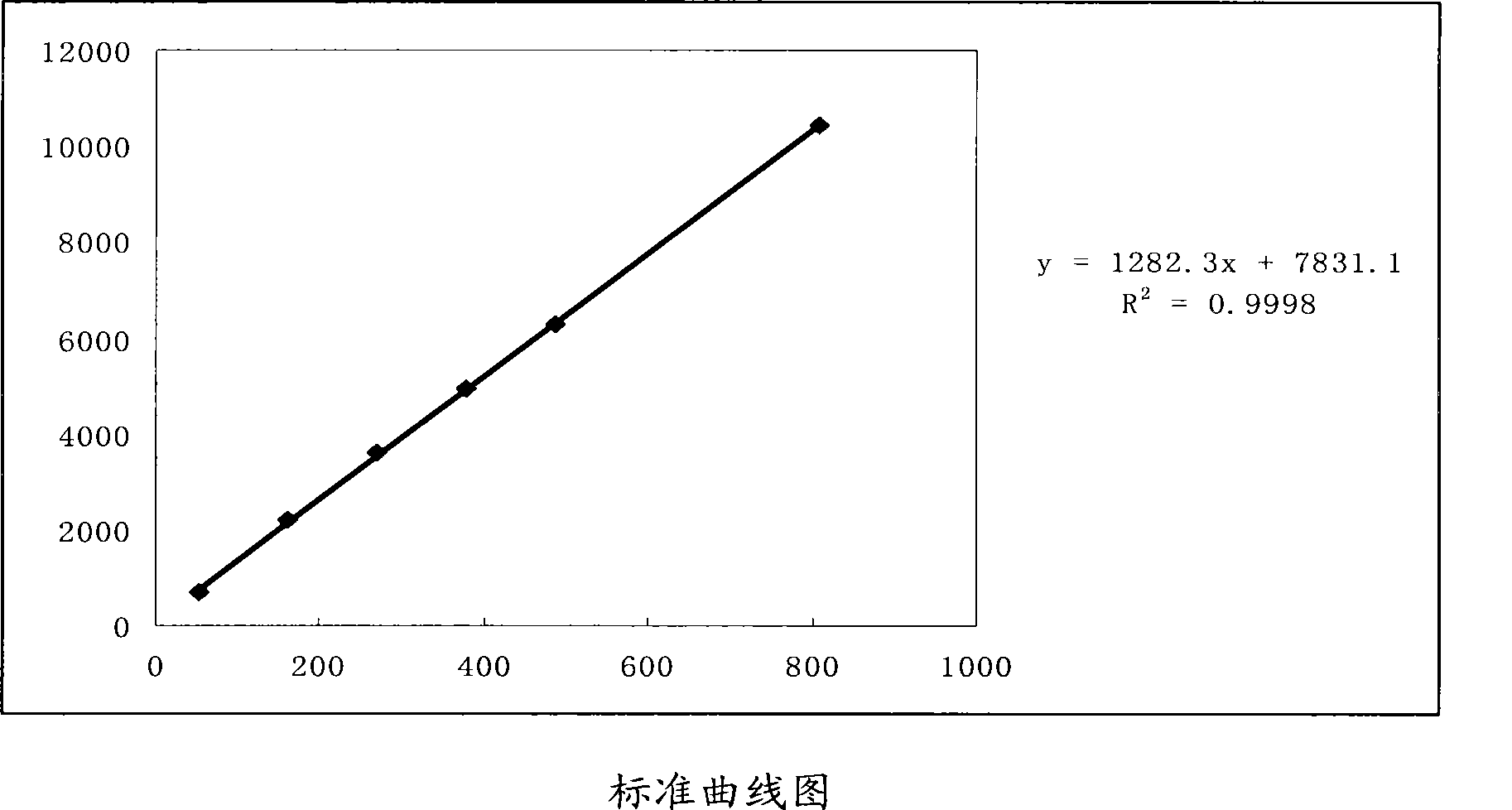
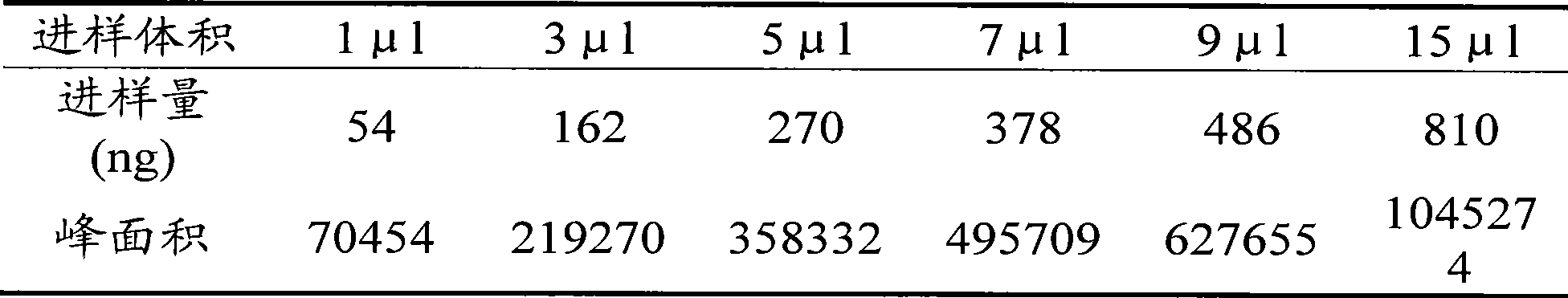
[0066] Chromatographic conditions: Agilent TC-C column 18 (250mm×4.6mm×5 μm); the mobile phase is acetonitrile-water (15:85); the detection wavelength is 248nm; the column temperature is 30°C. The number of theoretical plates is not less than 2000 based on the peak of ecdysterone.
[0067] Preparation of reference substance solution: Accurately weigh an appropriate amount of ecdysterone reference substance, add dilute ethanol to make a solution containing 30 μg per 1 ml, and obtain.
[0068] Preparation of the test solution: Take a sample of Houyanqing Oral Liquid, mix it evenly, accurately measure 25ml, put it in a 50ml measuring bottle, add ethanol to dilute to the mark, shake well, filter, and take the filtrate to obtain the final product.
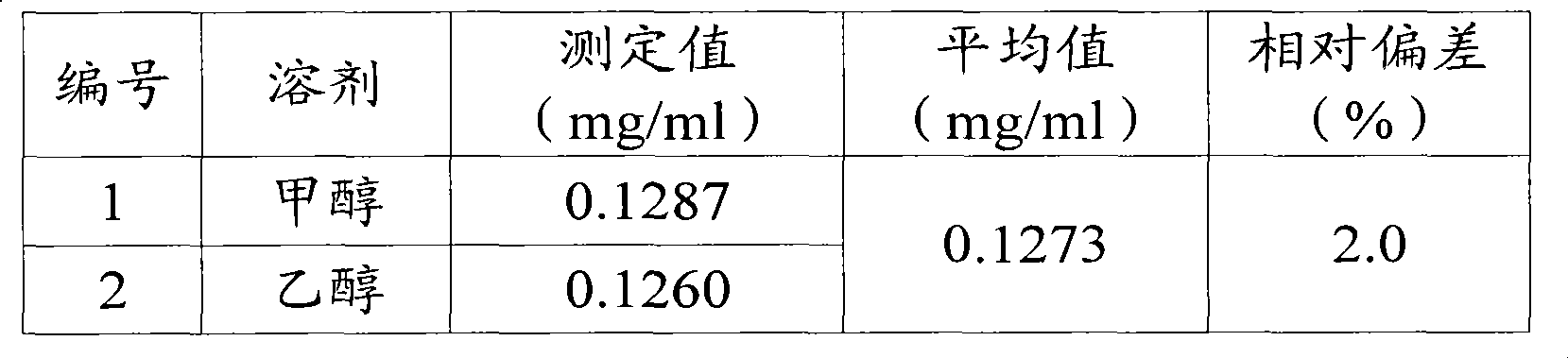
[0069] Deter...
Embodiment 2
[0075] According to the same method as in Example 1, the content of 10 batches of Achyranthes bidentata medicinal materials was measured, and each batch of samples was measured twice in parallel. The results are shown in Table 8.
[0076] Table 8 Determination results of ten batches of Achyranthes bidentata medicinal materials
[0077]
[0078]
[0079] According to the prescription quantity, 0.25 g of Achyranthes bidentata is needed to prepare 1 ml of Houyanqing Oral Liquid. After determination, the average content of 10 batches of Achyranthes bidentata medicinal materials is 0.04938% by weight. After testing 15 batches of Houyanqing oral liquid samples, the average content was 0.08383mg / ml, and the transfer rate was 67.9%.
[0080] After testing 15 batches of samples, the average content is 0.08383mg / ml, which is 0.058mg / ml converted by 70%, so the tentative content limit is: every 1ml of soil-containing Achyranthes bidentata is calculated as ecdysterone (C27H34O11), ...
Embodiment 3
[0082] Example 3 Quality Control in the Production Process of Houyanqing Oral Liquid
[0083] Add 20 times the amount of water to decoct the medicinal materials of the four flavors formula, and keep the volume of the decoction liquid constant during the decoction process. The concentration of ecdysterone in the decoction liquid was detected by high performance liquid chromatography. The detection conditions and operations are the same as in Example 1, wherein the decoction is taken, initially filtered, and 25ml is accurately measured, placed in a 50ml measuring bottle, diluted to the mark with ethanol, shaken up, filtered, and the subsequent filtrate is taken for sample detection. When detecting that the ecdysterone content in the sample reaches 0.01mg / ml, stop decocting. Each batch of test products was carried out twice, and in the products controlled by this method, the content of ecdysterone reached 0.075mg / ml. Under the conditions of this example, it usually takes only 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com