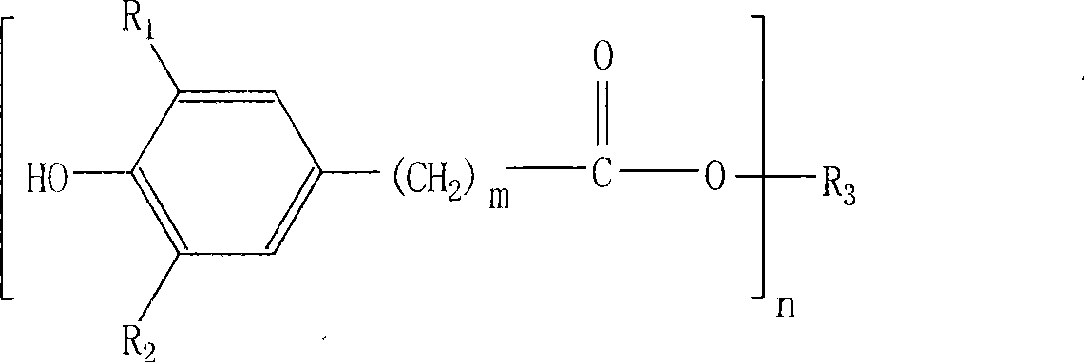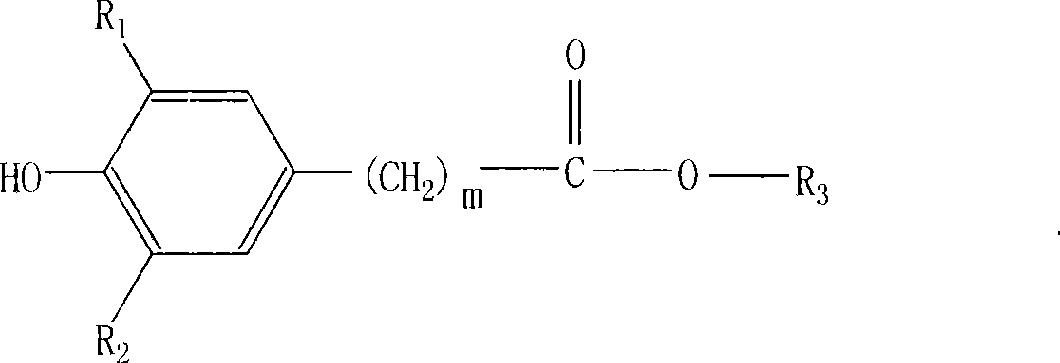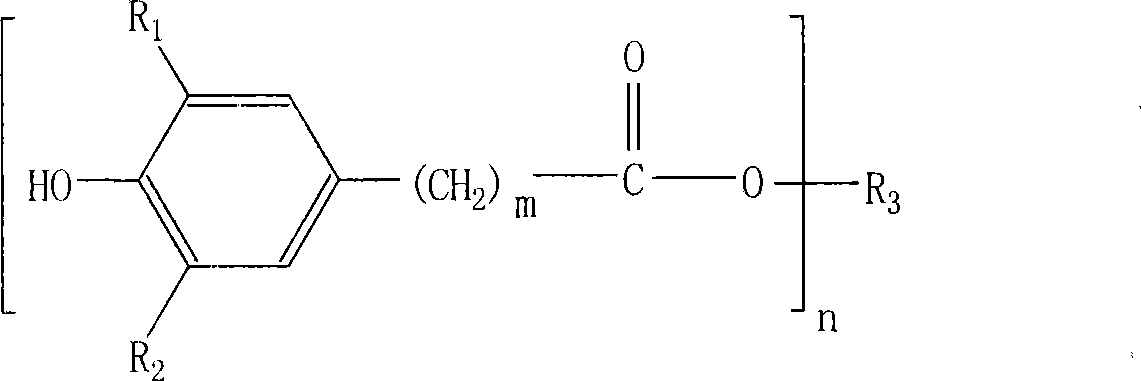Novel synthesis method for hindered phenol anti-oxidants
A technology for antioxidants and hindered phenols, which is applied in the synthesis of hindered phenolic antioxidants to achieve the effects of high product conversion rate, reduced waste discharge, and efficient catalytic reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Taking the typical product of antioxidant: tetra-[β-(3,5-di-tert-butyl, 4-hydroxyphenyl)propionic acid]pentaerythritol ester (antioxidant 1010) as an example to describe the implementation of the present invention .
[0035] In a four-necked flask equipped with a stirrer, a condenser and a heater, add 136 grams (1 mole) of pentaerythritol, 1460 grams (5 moles) of 3,5 methyl esters, and then add 7 grams of pre-configured composite catalysts (main The catalyst is diisooctyl dimaleate di-n-butyltin, the co-catalyst is tris-(2,4-di-tert-butylphenol) phosphite, the compound ratio is 6:1), and it is replaced with pure nitrogen. The air in the system depressurizes the system and heats it up. The first stage of low heat preservation reaction, the temperature is 140-165 ℃, the pressure is 5000-20000pa, the reaction time is 3 hours, and the by-product methanol is extracted. The reaction temperature of the second stage is 165-190°C, the pressure is 5000-100pa, and the...
Embodiment 2
[0038] Embodiment 2: (main catalyst and 3,5 methyl ester recycling experiment of recovery for the first time)
[0039] Stirrer is equipped with condenser, in the four-necked flask of heater, add 136 grams (1 mole) pentaerythritol, add the reclaimed 3,5 methyl esters (wherein contain main catalyst), 292 grams, add 1168 grams (4 moles again) ) of fresh 3,5 methyl ester, add 1 gram of cocatalyst tris-(2,4-di-tert-butylphenol) phosphite, replace the air in the clean system with pure nitrogen, decompress the system, and heat up. In the first stage of low heat preservation reaction, the temperature is 140-165 ° C, the pressure is 5000-20000 Pa, the reaction time is 3 hours, and the by-product methanol is extracted. In the second stage, the reaction temperature is 165-190°C, the pressure is 5000-100pa, the reaction time is 4 hours, and the by-product methanol is continuously extracted. The third stage: the temperature is 170-190°C, the pressure is 500-20pa. The 3,5 methyl esters are d...
Embodiment 3
[0042] Embodiment 3. (main catalyst and 3,5 methyl ester experiments after circulation many times)
[0043] The amount of the main catalyst contained in the 3,5 methyl ester after many cycles has been determined in advance, and the lost amount of the main catalyst is made up in the test. Stirrer is equipped with, condenser, in the four-necked flask of heater, add 68 grams (0.5 mole) pentaerythritol, add the 3,5 methyl ester 146 grams (0.5 mole) that contains procatalyst repeatedly, add 584 grams (2 mol) of fresh 3,5 methyl esters, add the amount of the main catalyst that is determined to be lost through testing, and this experiment determines to add 1 gram of the main catalyst dibutyltin dilaurate, and the cocatalyst di(octadecyl)dipropionate Esters 0.5 g. Replace the air in the clean system with pure nitrogen, depressurize the system, and raise the temperature. Continue the previous three-stage reaction operation, steam the 3,5 methyl ester and the main catalyst, break the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com