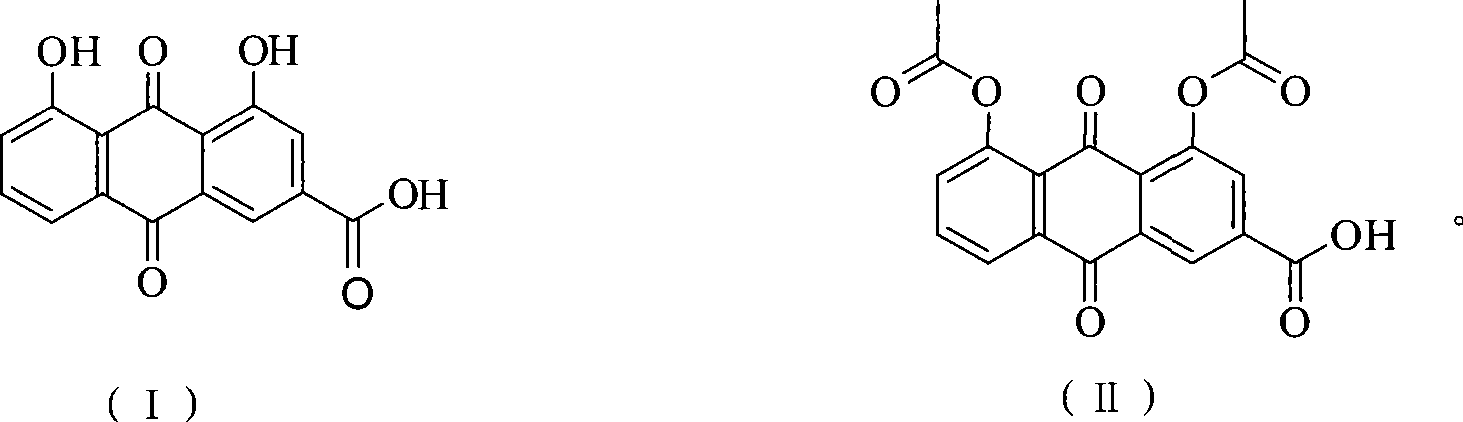
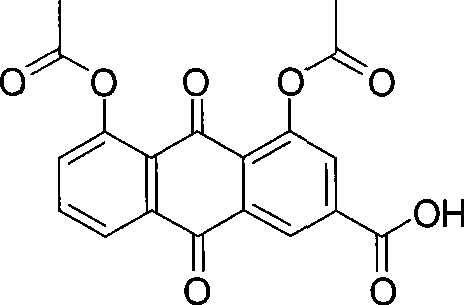
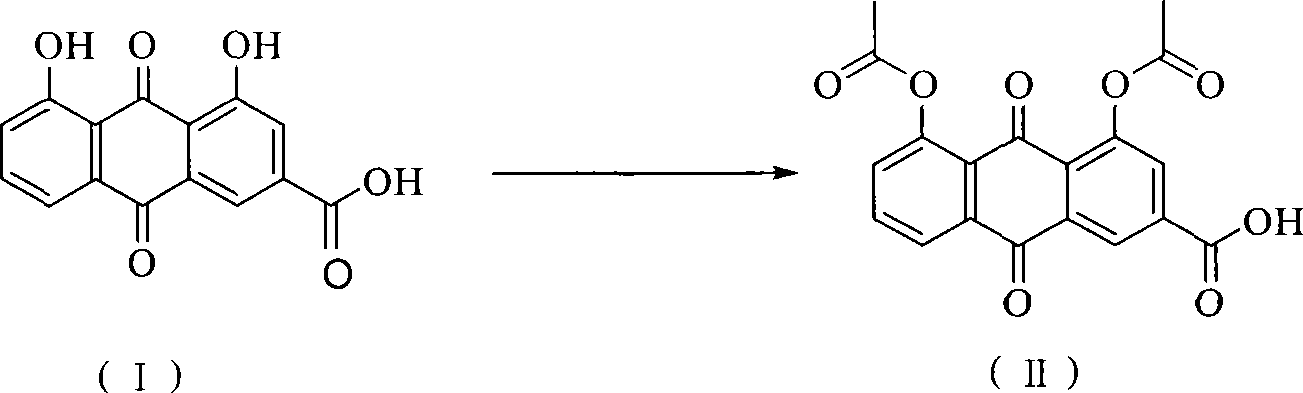
Method of preparing diacetyl rhein
A technology of diacetylrhein and rhein, applied in the preparation of carboxylic acid esters, preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of high production cost, achieve low production cost, short production cycle, and product yield and the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Rhein (5.64g, 20mmol), zinc trifluoromethanesulfonate (0.04g, 0.1mmol) and acetic anhydride (12.25g, 120mmol) were put into a 100ml reaction vessel, and the temperature was raised to 138°C for 1h under stirring. After the reaction, the temperature of the reaction solution was lowered to room temperature, and solids were precipitated, filtered, and the filter cake was recrystallized with acetic acid and dried to obtain diacetyl rhein (7.05 g). The HPLC purity was 99.5%, and the yield was 95.2%.
Embodiment 2
[0025] Rhein (5.64g, 20mmol), zinc trifluoromethanesulfonate (0.04g, 0.1mmol) and acetic anhydride (12.25g, 120mmol) were put into a 100ml reaction vessel, and the temperature was raised to 138°C for 2h under stirring. After the completion of the reaction, the temperature of the reaction solution was lowered to room temperature, a solid was precipitated, filtered, and the filter cake was recrystallized with acetic acid and dried to obtain diacetyl rhein (7.14 g). The HPLC purity was 99.7%, and the yield was 96.6%.
Embodiment 3
[0027] Put rhein (5.64g, 20mmol), zinc trifluoromethanesulfonate (0.04g, 0.1mmol) and acetic anhydride (8.17g, 40mmol) into a 50ml reaction vessel, and heat to 138°C for 0.5h under stirring. . After the completion of the reaction, the temperature of the reaction solution was lowered to room temperature, a solid was precipitated, filtered, and the filter cake was recrystallized with acetic acid and dried to obtain diacetyl rhein (7.10 g). The HPLC purity was 99.4%, and the yield was 95.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com