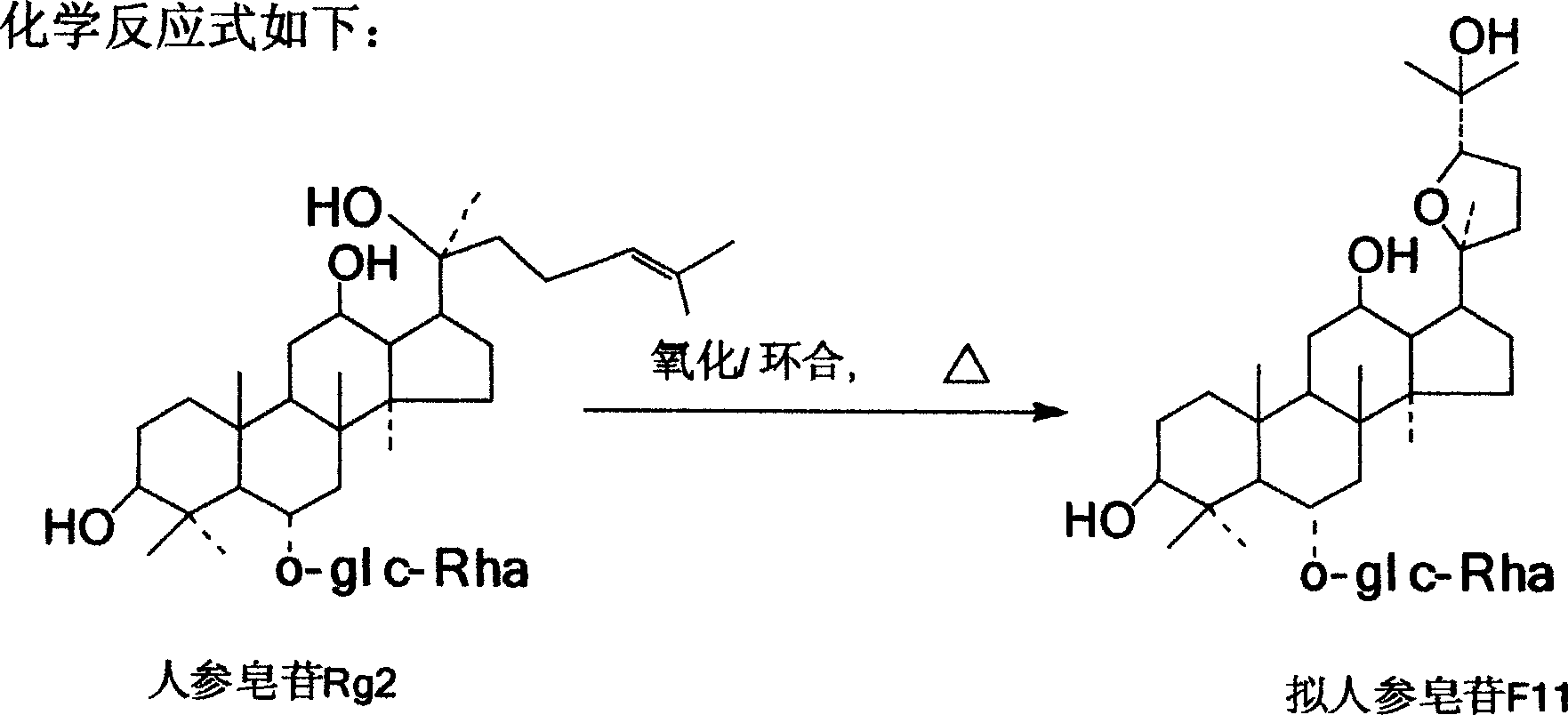
Semi-synthetic method of pseudo-ginsenoside F11
A technology for simulating ginsenosides and ginsenosides, applied in the directions of steroids, organic chemistry, etc., can solve the problems such as no reports on the semi-synthetic method of ginsenoside F11, and achieve the effects of significant curative effect, improvement of mood, and ease of reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] a. Add 5.0g ginsenoside Rg2 to 50ml 1,4-dioxane, add concentrated H 2 SO 4 Adjust the pH value to 3-5, add 20ml of p-chloroperbenzoic acid dropwise under stirring, heat and reflux at 85°C for 20 minutes, after cooling, adjust the pH to 7.0 with 0.1M NaOH aqueous solution, filter, and concentrate the filtrate to dryness to obtain Crude pseudo-ginsenoside-F11: 3.0g;
[0015] b. Purification of pseudo-ginsenoside F11: take crude pseudo-ginsenoside F11 and carry out silica gel column chromatography, the eluent is n-butanol: ethyl acetate: water = 65:35:10, and n-butanol solvent extraction method can also be used Purification was carried out to obtain pure pseudoginsenoside-F11: 2.5g.
[0016] Ginsenoside Rg2 can be obtained by conventional methods in the prior art, for example:
[0017] Take 5.0g ginsenoside Re, add 22g NaOH, dissolve in 50ml 1,2-propanediol, heat to reflux for 5h, and cool at room temperature. After adding 1.5 times of water to dilute, use concentrated...
Embodiment 2
[0019] Get 5.0g ginsenoside Rg2 and add in 50ml cyclohexane, add concentrated H 2 SO 4 Adjust the pH to 5, add m-chloroperbenzoic acid 20ml dropwise under stirring, heat and reflux at 85°C for 30 minutes, after cooling, adjust the pH to 7.0 with 0.1M NaOH aqueous solution, filter, and concentrate the filtrate to dryness to obtain crude Ginsenoside F11: 3.1g.
Embodiment 3
[0021] Take 5.0g ginsenoside Rg2 and add it to 50ml 1,4-dioxane, add concentrated H 2 SO 4 Adjust the pH to 4, add 20ml of peracetic acid dropwise under stirring, heat and reflux at 80°C for 20 minutes, after cooling, adjust the pH to 7.0 with 0.1M NaOH aqueous solution, filter, and concentrate the filtrate to dryness to obtain crude pseudoginsenoside F11 : 3.7g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com