Novel morphine derivatives
A compound and pharmaceutical technology, applied in the field of new morphine derivatives, can solve problems such as addiction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
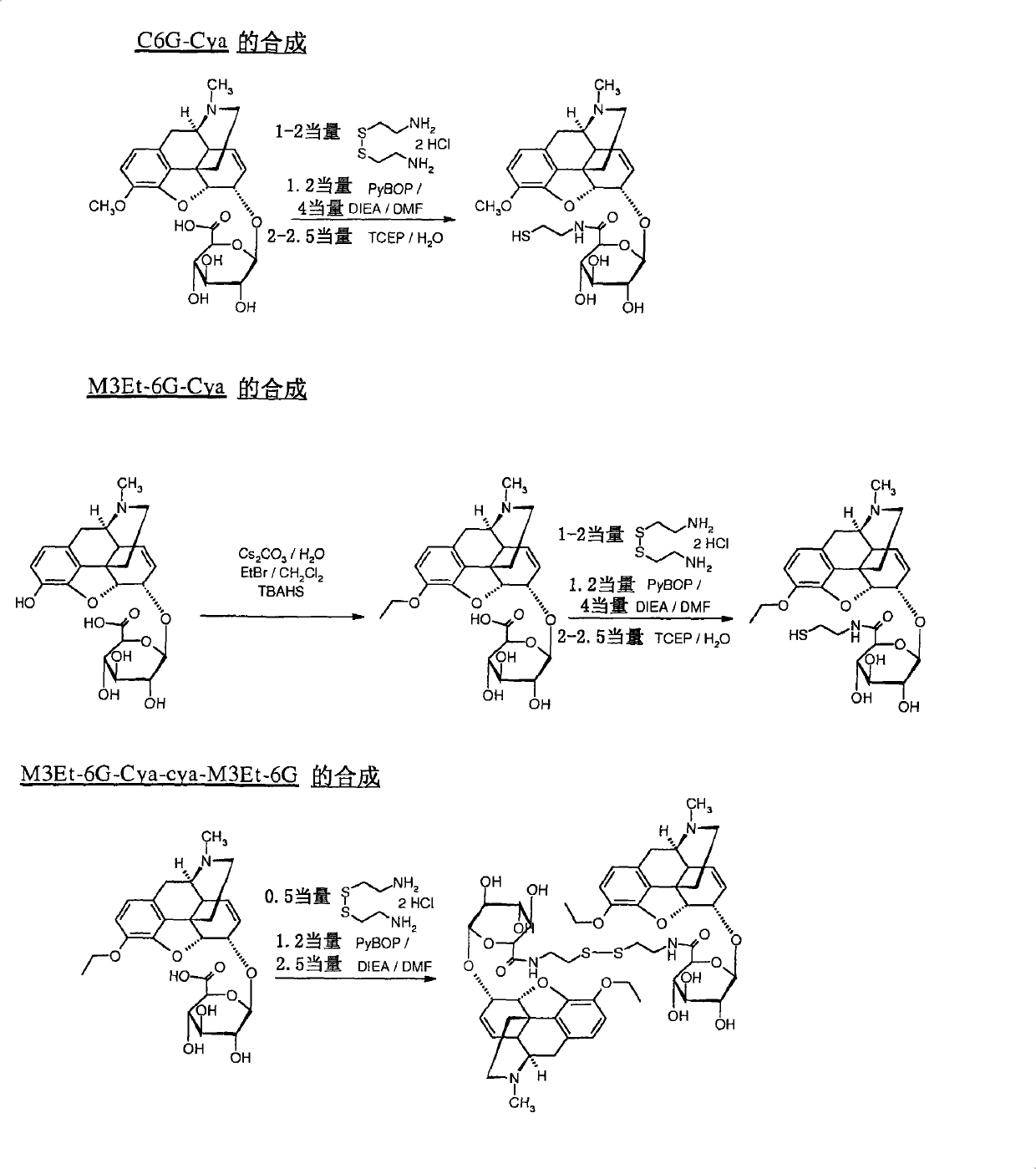
[0096] Example 1: Synthesis of C6G-Cya
[0097] In the reactor, 2 molar equivalents of cysteamine were dissolved in anhydrous dimethylformamide (DMF) at a concentration of 138 g / l. After dissolution in dry DMF, 1 molar equivalent of commercially available codeine-6-glucuronide·TFA was added at a concentration of 47 g / l.
[0098] 4 molar equivalents of diisopropylethylamine (DIEA) were added and the reactor was placed in an ice bath (0°C). 1.2 molar equivalents of benzotriazol-1-yl-oxyl-tris-pyrrolidinylphosphonium hexafluorophosphate (PyBOP) dissolved in DMF at a concentration of 225 g / l in advance was added dropwise to the reaction mixture, It was then stirred at room temperature for 3 hours.
[0099] The disulfide bridges were then reduced by adding 2.5 molar equivalents of tris(2-carboxyethyl)phosphine (TCEP) predissolved in water / TFA 0.1% at a concentration of 214 g / l. After 2 hours of reaction, the product was purified by preparative HPLC.
[0100] 10.3 mg of C6G-cy...
Embodiment 2
[0101] Example 2: Synthesis of M3Et-6G-cya
[0102] Synthesis of M3ET-6G:
[0103] In the reactor, 1 molar equivalent of morphine-6-glucuronide·2H 2 O(M6G) was poured and dissolved in water to a concentration of 100g / l. 5 equivalents of cesium carbonate were added, and the mixture was stirred at room temperature for 5 minutes.
[0104] An equal volume of dichloromethane to water was added to the mixture. 5 equivalents of ethyl bromide and 2 equivalents of tetrabutylammonium hydrogensulfate (TBAHS) were added continuously. Stirring was continued at room temperature for 72 hours. The product was purified by preparative HPLC.
[0105] Nuclear Overhouse effect (NOE) experiments by proton nuclear magnetic resonance (NMR) indicated that the oxygen atom at position 3 of the morphine derivative bears an ethyl group. In fact, the controlled irradiation of the ethyl group affects the signal of the phenolic aromatic proton.
[0106] Obtained 45.8 mg of M3Et-6G: [M+H] + =490-M ...
Embodiment 3
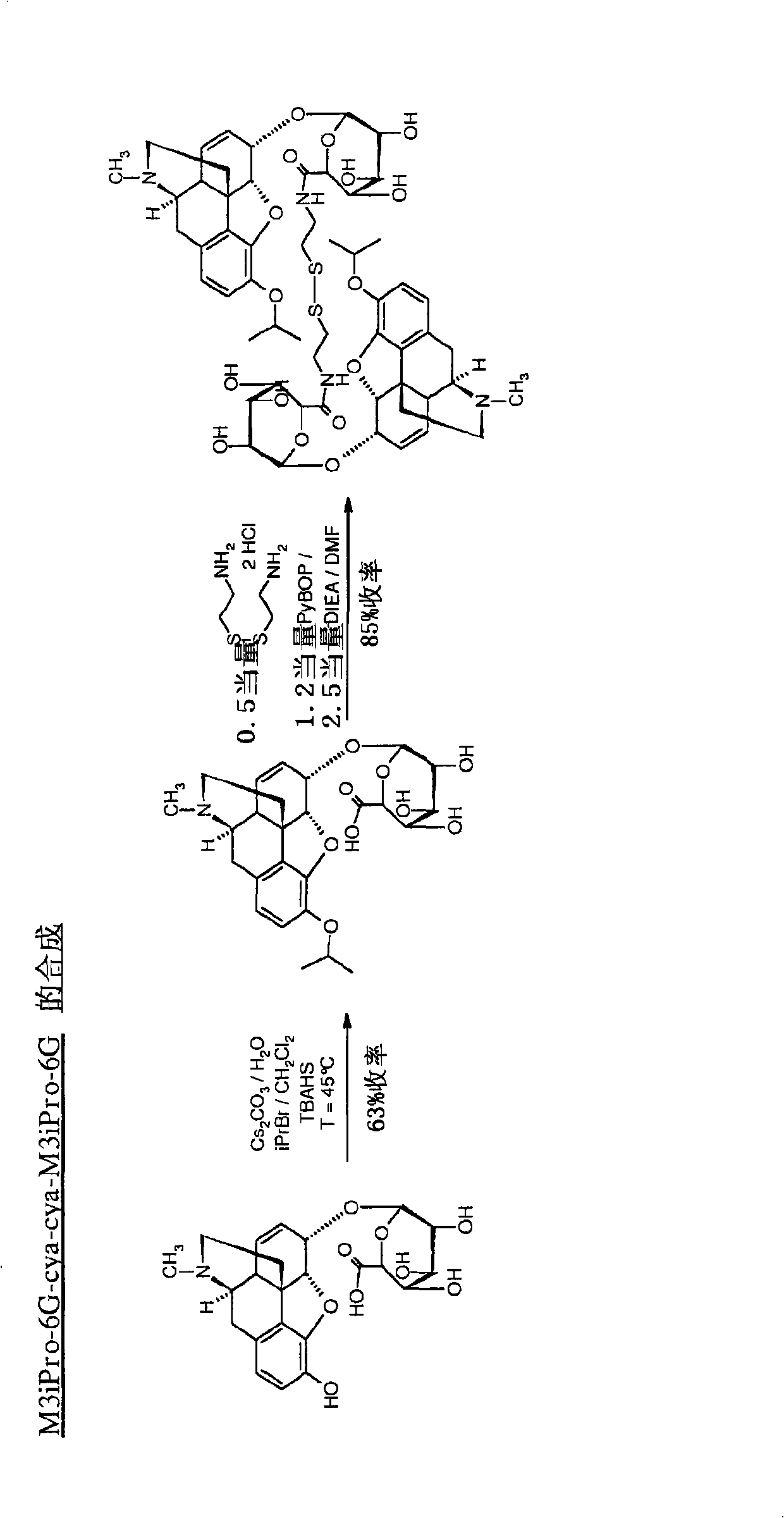
[0111] Example 3: Synthesis of M3Et-6G-cya-cya-M3Et-6G
[0112]In the reactor, 1 molar equivalent of cysteamine dihydrochloride was dissolved in DMF at a concentration of 86 g / l. 2 molar equivalents of M3Et-6G-cya, previously dissolved in DMF at a concentration of 97 g / l, were added. Then 5 molar equivalents of pure DIEA were introduced and the mixture was cooled to 0 °C in an ice bath. 2.4 molar equivalents of PyBOP dissolved in DMF at a concentration of 22.9 g / l were added dropwise. Stirring was continued at room temperature for 3 hours. The product was then purified by preparative HPLC.
[0113] Obtained 40.5 mg of M3Et-6G-cya-cya-M3Et-6G dimer: [M+H] + =1096-M TFA = 1322 - Purity: 95% - Yield = 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com