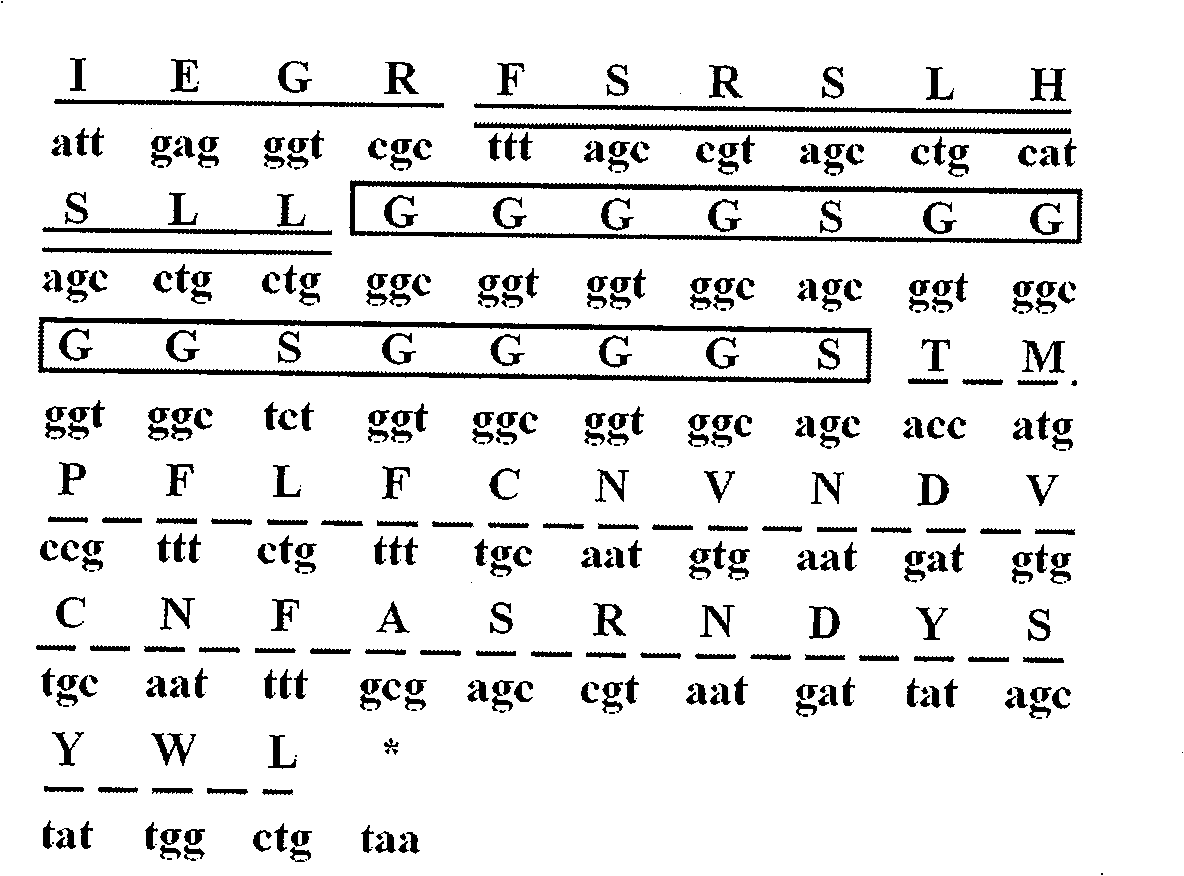Recombination NuBCP-9 and Tumstatin(74-98) antitumor fusion polypetide
A fusion peptide, anti-tumor technology, applied in the direction of anti-tumor drugs, hybrid peptides, drug combinations, etc., can solve problems such as complex mechanisms and enhanced anti-tumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1. Fusion peptide sequence design and spatial structure prediction
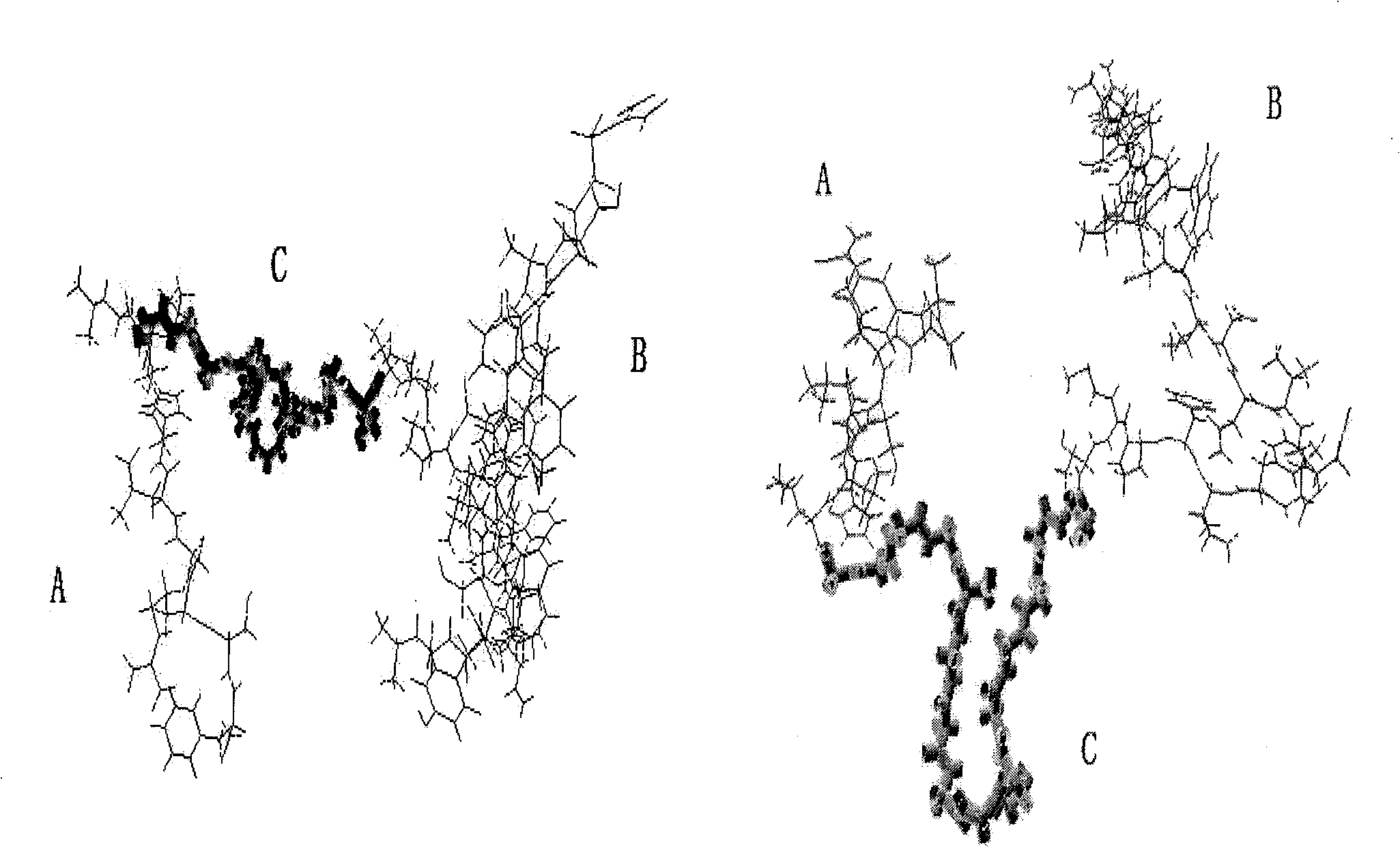
[0027] According to the amino acid sequence of NuBCP-9 and Tumstatin (74-98), a flexible peptide (G 4 S) 3 The nucleic acid sequence was designed according to the preferred codons of Escherichia coli, and adjusted appropriately according to the principles of PCR primer design ( figure 1 ). In order to determine whether the spatial structure after connection will affect the function of the respective peptides, we used the molecular simulation software MOE (molecular operating environment) to perform homology modeling predictions on the fusion peptide, using the protein with PDB number 1F3R.B as a template . The environment used in the space simulation is the physiological pH value water phase, and other settings are default. Forecast results such as figure 2 , it can be seen from the figure that its spatial structure can be well maintained and meets the design requirements.
Embodiment 2
[0028] Example 2. Construction of high-efficiency expression vectors
[0029] (1) Based on the amino acid sequences of NuBCP-9 and Tumstatin (74-98), the nucleic acid sequences were designed in comparison with the preferred codons of Escherichia coli, and were appropriately adjusted according to the principles of PCR primer design, and four primers were designed, respectively:
[0030] P1f::GAC GAATTC TTTAGCCGTAGCCTGCATAGCCTGCTGGGCGGTGGTGG
[0031] P2f: CCTGCTGGGCGGTGGTGGCAGCGGTGGCGGTGGCTCTGGTGGCGGTGGCAGCACCATGC
[0032] P3r: GCAAAATTGCACACATCATTCACATTGCAAAACAGAAACGGCATGGTGCTGCCACCGCC
[0033] P4r: CGC AAGCTT TTACAGCCAATAGCTATAATCATTACGGCTCGCAAAATTGCACACATCAT
[0034] At the same time, the restriction enzyme sites EcoR I and HindIII, and the protease Factor Xa site sequence were introduced.
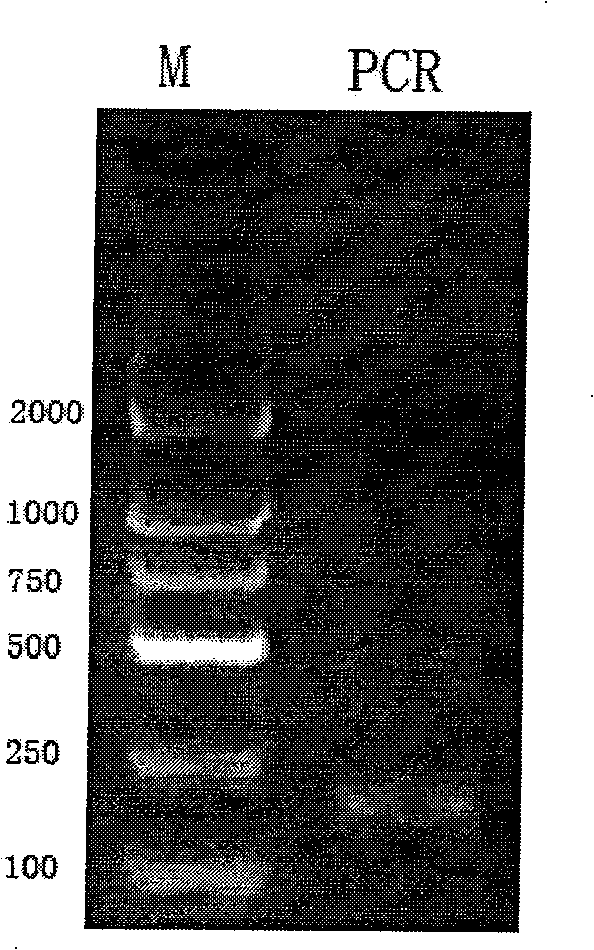
[0035] (2) The NuBCP-9 / Tumstatin (74-98) fusion peptide gene was amplified by two-step SOE method.
[0036] The first step of PCR: using P2f and P3r as templates, adding Taq enzym...
Embodiment 3
[0040] Example 3. Expression and purification of fusion peptide NuBCP-9 / Tumstatin (74-98)
[0041] (1) Transform the positive cloned plasmid with correct sequencing into Escherichia coli expression strain BL21(DE3), insert the obtained transformants into LB liquid medium, culture at 37°C overnight, dilute 1:50 the next day and expand culture at 37°C for 3 hours , adding 0.5mM IPTG to induce expression at 30°C (attached Figure 6) , the expression form was analyzed by 12% SDS-PAGE, and the expression amount was analyzed by electronic scanning.
[0042] (2) The fusion protein TrxA-NuBCP-9 / Tumstatin (74-98) was expressed in a soluble form. After a large amount of expression, the bacteria were collected by centrifugation and resuspended with NTA-Resin Buffer. Collect the supernatant.
[0043] (3) Purification to obtain fusion protein
[0044] The supernatant was separated and purified by affinity chromatography column NTA Resin to obtain the fusion protein TrxA-NuBCP-9 / Tumstati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com